The glaucoma pipeline is promising for both outflow and nerve protection. This is good news for patients who are already benefitting from the lastest round of glaucoma agents, which included an entirely new class of drugs, the rho kinase inhibitors. Netarsudil is a rho-kinase inhibitor used nightly, packaged by itself (Rhopressa; Aerie Pharmaceuticals) or combined with latanoprost (Rocklatan; Aerie Pharmaceuticals). The combination of these agents was conceived to leverage their complementary modes of action in lowering eye pressure. Latanoprost increases uveoscleral outflow and has some effect on the trabecular meshwork (TM) extracellular matrix, while netarsudil decreases epsicleral venous pressure, decreases trabecular stiffness, and may decrease aqueous production, among other mechanisms of action. Thus, there are several mechanisms in a single once-a-day bottle. In the future, “mechanisms per daily drop” may be a new way of thinking about glaucoma medication.
Netarsudil is also showing promise for more than simple primary glaucoma, including for corticosteroid-induced glaucoma and, perhaps, glaucoma associated with anti-VEGF drugs. The addition of nitric oxide holds the added potential of altering the TM to alleviate the high-pressure–causing stiffness of the TM, which seems to characterize high-pressure glaucoma. This class of medicine also might increase blood flow in the eye, although this remains to be proven.
Vyzulta (Bausch + Lomb), a combination of nitric oxide and latanoprost, is dosed once daily. It should be noted that the Vyzulta dose of latanoprost is substantially more than is seen in generic latanoprost; there is some evidence that this is not having any effect upon intraocular pressure (IOP). Adding nitric oxide to numerous molecules holds a great deal of promise for increasing efficacy and therapeutic potency. New formulations are anticipated over the next few years.
New candidate drugs are in varying stages of development, but while they may put in appearances as single posters at ARVO or at the Trabecular Meshwork Study Club, which meets annually in association with the American Society of Cell Biology, in many instances, they are not yet publicly announced due to regulatory and intellectual property concerns. The addition of nitric oxide to bimatoprost has been studied recently and appears to be very effective, although it is not yet commercially available.1
Pipeline drugs are valuable because, in addition to introducing promising new treatments, they give patients hope for improvements in care. It is prudent to start slowly and be aware of side effects and how to cope with them as new agents are used in the clinic. We are entering an era with new potential treatment modalities; the old adage for glaucoma patients that “You will be on drops for the rest of your life” is no longer true when, for example, stem cells can be added to the TM to restore function.2 Younger patients will likely be on different medications 10 years from now.
Some glaucoma specialists believe that in 10 years, medications will include current conventional approaches of pressure-lowering medications, plus the neuroprotecting or neuroregenerating medications that were introduced this past year, plus an emerging third class, “nutraceuticals,” as there is growing evidence that a wide variety of food-based agents, such as goji berries,3 resveratrol,4 curcumin in turmeric,5 gingko preparations,6 and other alternative nutraceuticals that are unlikely to be developed by large pharmaceutical companies may play a role in patient management. And, of course, while nutraceuticals and alternative therapies often draw patient interest, and may play a role in overall care, they should never be used in lieu of conventional therapy. Concern about using these compounds in a rational manner may require glaucoma specialists to gain an understanding of naturopathy and nutritional information. Another category of treatment is the combination of surgical devices or laser treatment in novel ways with medications. This may work in ways that cannot be achieved by either treatment alone. Drug-delivery devices are rapidly advancing, and collagen drug-delivery devices are just starting to be considered.
Appropriate Patient Education
Patients must be informed of and consent to the level of evidence supporting their treatment. Glaucoma specialists wishing to provide a full spectrum of therapies should enable patients to make choices while also ensuring their safety. Glaucoma is uniquely problematic in this regard because it is rarely manifested to the patient in a notable way. Physicians need to follow the dictum to “do no harm.” It is my practice to document that the patient agrees not to deviate from standard therapy before discussing in detail the merits of adding nontraditional medications. Some patients may be more compliant than others when their physician embraces a more holistic approach.
Development Timeline
In discussing drugs in the near pipeline, we must bear in mind the long lead time it may take to develop a new glaucoma medicine, which may or may not ultimately be successfully introduced into the marketplace. It was almost 20 years from the time that Dr. Mortimer Civan first described a pressure-lowering response elicited by adenosine receptors at ARVO,9 and ultimately the drug failed to become a commercial success.10 The theoretical basis for the adenosine class was good, but the clinical trial did not pan out. It is the nature of drug development to develop, refine, and sometimes reject drugs when their performance isn’t competitive with the efficacy of “gold standard” therapies, such as timolol or latanoprost for glaucoma. These gold standard therapies, of course, still have serious adverse effects, and it is much more difficult to find drugs superior to the “gold standard” with less serious side effects. The lack of serious side effects of the new drugs, such as latanaprostene bunod and netarsudil, needs to be highlighted when comparing them to drugs such as those held to be the gold standard. Netarsudil had no serious adverse events in clinical trials.11
Preservative-Free Drugs
The pipeline, then, involves looking for superior efficacy with increased safety and means of obtaining good compliance. With the advent of bottles that can support preservative-free medication, we can hope that a future trend towards minimizing or eliminating preservatives will continue. At this writing, there are 2 preservative-free prostaglandins available, one in a bottle (Xelpros; Sun Ophthalmics) and one as a single-unit dose (Zioptan; Akorn). Preservative-free timolol (timolol 0.25% and 0.5% in Occudose from Bausch + Lomb) and timolol combined with dorzolamide (Cosopt PF; Akorn) are also available. Brimonidine is available in a non-benzalkonium chloride (BAK) formula (Alphagan P; Allergan), as is a prostaglandin without BAK but with preservative (Travatan Z; Novartis). The toxicity of BAK was well documented in the early 1980s.12 The literature on the toxicity of preservatives continues to expand. In the author’s view, BAK is toxic to eye tissues and leads, in many cases, to thickened conjunctiva and Tenon’s capsule, which compromises glaucoma surgery. Expect additional glaucoma preparations to become available in the future.
Promising New Compounds
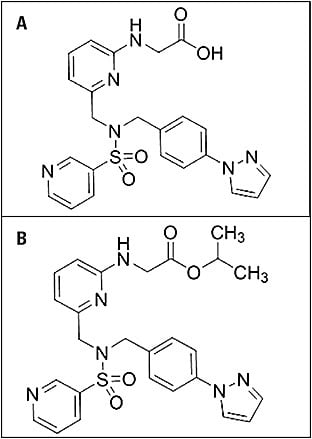
Omidenepag isopropyl (DE-117; Santen) shows promise. This molecule (Figure 1) binds selectively to the prostaglandin EP2 receptors while not being similar to a prostaglandin itself.13-15 This may avoid the side effects associated with prostaglandin use, such as hyperemia, lash growth, loss of orbital fat, and iris color change. It is still too early in development to project when this might be available in the United States, although it has recently been approved in Japan with the commercial name Eyebelis to treat ocular hypertension and glaucoma. Data were presented at the 2019 Trabecular Meshwork Study Club that could pave the way for future discussion.16
As noted, adding nitric oxide to other molecules seems to increase their potency. There is early promise with the addition of nitric oxide to bimatoprost. Indeed, most molecules, not just prostaglandins and glaucoma drugs, may be enhanced by the addition of nitric oxide. The phase 2 multicenter Dolomites trial, a dose-ranging study investigating a second-generation nitric oxide-donating bimatoprost analog NCX 470 (Nicox), is ongoing (NCT03657797). Nicox recently reported that NCX 470 met its primary endpoint of noninferiority to latanoprost.17
Three other compounds are worth special mention. The modulation of IOP by ATP-sensitive potassium channel openers has been investigated at the Mayo Clinic.18 A candidate compound shows substantial promise. Study of the autotaxin-lysophosphatidic acid has shown substantial promise with glaucoma candidates.1,19-23 A promising IOP-lowering drug has been developed by Aerpio. This is a novel activator of Tie2 known as AKB-9778.
Drug-delivery changes are coming, including both the intraocular pellet placed in the anterior chamber developed by Allergan (Bimatoprost SR) and a drug-eluting product from Glaukos (iDose). Neither of these are FDA approved yet. Pellets in the posterior chamber are going to be needed if peptide molecules are going to release either small molecules or, more likely, larger peptide molecules. Many efforts in posterior-segment delivery are under way. There are a number of intriguing drug-delivery devices that have not yet been publicly announced.
The Far Glaucoma Pipeline
The glaucoma pipeline is robust. Public discussion is prevented due to recent changes in how intellectual property is handled.24 Not every new compound will be a success, and some will fail at later stages of testing. However, it is time to stop telling patients that they will be on the same medications for the rest of their lives. In fact, it’s a good idea to try the drugs that have become available within the past year; they have merit, and more new drugs will be coming.
Ultimately, success in treating glaucoma with new agents depends on understanding the nature of glaucoma. Genetics prove that primary open-angle glaucoma is actually many different diseases. Numerous monogenetic causes of primary open-angle glaucoma have been discovered; a variety of mutations have the common pathway of raised eye pressure and loss of the ability of the TM to maintain homeostasis.25
Understanding the biology of the TM, Schlemm’s canal, and the optic nerve in terms of extracellular matrix biosynthesis and degradation, as well as the physiologic process of pore formation and the microanatomy of the outflow pathway, will lead to more candidate compounds.26,27 Studying the biologic effects of the new rho kinase class of which Netarsadil is the first commercial example in the United States is likely to lead to many more candidate compounds.
Future Discovery
There are many interconnected pathways associated with the homeostatic response in the TM that maintains eye pressure. Understanding extracellular matrix turnover and modification in the TM by looking at the proteins in the high-flow and low-flow areas of the TM is promising. Knowing where these high-flow and low-flow areas are located (Figure 2) is important for the implantation of surgical devices in the canal. Promising findings have been reported at ARVO, ISER, and the Trabecular Meshwork Study Club that are likely to expand the glaucoma pipeline very quickly. It is the author’s opinion that the comparison of the proteins in the high-flow and low-flow areas is the most promising.28 GP

References
- Impagnatiello F, Bastia E, Almirante N, et al. Prostaglandin analogues and nitric oxide contribution in the treatment of ocular hypertension and glaucoma. Br J Pharmacol. 2019;176(8):1079-1089.
- Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009;88(4):747-751.
- Morrone LA, Rombola L, Adornetto A, Corasaniti MT, Russo R. Rational basis for nutraceuticals in the treatment of glaucoma. Curr Neuropharmacol. 2018;16(7):1004-1017.
- Avotri S, Eatman D, Russell-Randall K. Effects of resveratrol on inflammatory biomarkers in glaucomatous human trabecular meshwork cells. Nutrients. 2019;11(5).
- Davis BM, Pahlitzsch M, Guo L, et al. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci Rep. 2018;8(1):11066.
- Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. 2012;18:390-402.
- Daiber A, Xia N, Steven S, et al. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci. 2019;20(1).
- Treuer AV, Gonzalez DR. Nitric oxide synthases, S-nitrosylation and cardiovascular health: from molecular mechanisms to therapeutic opportunities (review). Mol Med Rep. 2015;11(3):1555-1565.
- Avila MY, Stone RA, Civan MM. A(1)-, A(2A)- and A(3)-subtype adenosine receptors modulate intraocular pressure in the mouse. Br J Pharmacol. 2001;134(2):241-245.
- Rich CC, Albers DS, Gow JA, Baumgartner RA. Targeting the adenosine A1 receptor in the eye with trabodenoson, an adenosine mimetic. In: Samples JR, Knepper PA, eds. Glaucoma Research and Clinical Advances 2018 to 2020. Vol 2. Amsterdam, The Netherlands: Kugler Publications; 2018:237-252.
- Decalmer PB, Chatterjee SS, Cruickshank JM, Benson MK, Sterling GM. Beta-blockers and asthma. Br Heart J. 1978;40(2):184-189.
- Samples JR, Binder PS, Nayak S. The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp Eye Res. 1989;49(1):1-12.
- Klimko PG, Sharif NA. Discovery, characterization and clinical utility of prostaglandin agonists for the treatment of glaucoma. Br J Pharmacol. 2019;176(8):1051-1058.
- Sharif NA. Glaucomatous optic neuropathy treatment options: the promise of novel therapeutics, techniques and tools to help preserve vision. Neural Regen Res. 2018;13(7):1145-1150.
- Kirihara T, Taniguchi T, Yamamura K, et al. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59(1):145-153.
- Sharif NA. Eybelis (omidenepag isopropyl; DE-117) conventional and uveoscleral outflow-promoting non-prostaglandin EP2-receptor agonist drug for glaucoma treatment. Abstract presented at: 18th annual Trabecular Meshwork Study Club meeting; December 2019; Washington, DC.
- Top line results from glaucoma Dolomites phase 2 trial show Nicox’s NCX 470 meets primary endpoint and demonstrates statistical superiority vs latanoprost [press release]. Available at: https://www.nicox.com/news-media/top-line-results-from-glaucoma-dolomites-phase-2-trial-show-nicoxs-ncx-470-meets-primary-endpoint-and-demonstrates-statistical-superiority-vs-latanoprost/
- Chowdhury UR, Dosa PI, Fautsch MP. Modulation of intraocular pressure by ATP sensitive potassium channel openers. In: Samples JR, Knepper PA, eds. Glaucoma Research and Clinical Advances 2018 to 2020. Vol 2. Amsterdam, The Netherlands: Kugler Publications; 2018:201-220.
- Nagano N, Honjo M, Kawaguchi M, et al. Development of a novel intraocular-pressure-lowering therapy targeting ATX. Biol Pharm Bull. 2019;42(11):1926-1935.
- Ho LTY, Osterwald A, Ruf I, et al. Role of the autotaxin-lysophosphatidic acid axis in glaucoma, aqueous humor drainage and fibrogenic activity. Biochim Biophys Acta Mol Basis Dis. 2020;1866(1):165560.
- Igarashi N, Honjo M, Kurano M, et al. Increased aqueous autotaxin and lysophosphatidic acid levels are potential prognostic factors after trabeculectomy in different types of glaucoma. Sci Rep. 2018;8(1):11304.
- Honjo M, Igarashi N, Kurano M, et al. Autotaxin-lysophosphatidic acid pathway in intraocular pressure regulation and glaucoma subtypes. Invest Ophthalmol Vis Sci. 2018;59(2):693-701.
- Honjo M, Igarashi N, Nishida J, et al. Role of the autotaxin-LPA pathway in dexamethasone-induced fibrotic responses and extracellular matrix production in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2018;59(1):21-30.
- Noonan W. Patents in an age of innovation. In: Samples JR, Ahmed IIK, eds. Current Developments in Glaucoma Surgery and MIGS. Amsterdam, The Netherlands: Kugler Publications; 2020:39-54.
- Acott TS, Kelley MJ, Keller KE, et al. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014;30(2-3):94-101.
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86(4):543-561.
- Yang YF, Sun YY, Acott TS, Keller KE. Effects of induction and inhibition of matrix cross-linking on remodeling of the aqueous outflow resistance by ocular trabecular meshwork cells. Sci Rep. 2016;6:30505.
- Vranka JA, Staverosky JA, Reddy AP, et al. Biomechanical rigidity and quantitative proteomics analysis of segmental regions of the trabecular meshwork at physiologic and elevated pressures. Invest Ophthalmol Vis Sci. 2018;59(1):246-259.









