The pathophysiology of glaucoma still is not fully understood, and intraocular pressure (IOP) remains the only treatable risk factor.1 Despite the ever-growing number of IOP-lowering medications and procedures, innovation has been slower when it comes to IOP-measurement techniques, and the gold standard technique has essentially remained the same since 1950, when Goldmann applanation tonometry (GAT) was introduced.2 However, this technique is widely regarded as imperfect, and studies have pointed out its flaws in both design and concept. Not only is GAT relatively imprecise, but also its instant nature fails to reflect the complexity of real-life IOP variations.3-6 Over the last decades, ample evidence has shown that individuals’ IOP measurements are far from static and fluctuate widely over the course of 24 hours and through the year.7-12 From these observations stemmed a need to develop practical and accurate ways to observe continuous IOP changes. Also, while the use of diurnal tension curve further confirmed this necessity, it also highlighted the need for a more reproducible, practical, and continuous 24-hour measurement technique.13-15 This article reviews existing and emerging technologies that may one day supplant in-clinic tonometry, and explores the hidden benefits of such devices (Table 1).
| Characteristic | iCare Home Tonometer | TriggerFish Contact Lens Sensor | Eyemate Implantable Sensor |
|---|---|---|---|
| Size | External (11 cm x 8 cm) | 14.1 mm | 11.3 mm (sulcus device); 7.8 mm x 3.8 mm (suprachoroidal) |
| Data Availability | Patient blinded to data; downloaded from device at medical appointment | Patient blinded to data; downloaded during contact lens removal appointment | Data displayed to patient; wirelessly transferred to the specialist |
| Measurement Frequency | Patient triggered | 10 Hz for 30 seconds every 5 minutes | Patient triggered |
| Maximum Recording Period | As needed | 24 hours | Permanent |
| Nocturnal/Autonomous Measurements | Not possible | Yes/fully autonomous | Possible with specific accessories |
| Real-Life Activity Measurements | Not possible | Possible in some cases (as allowed by patient-worn electronic apparatus) | Possible in most cases |
| Patient Advantages | NoninvasiveVisible measurements Used as needed |
Relatively noninvasive One-time investigation |
Permanent Contactless measures Visible measurements Automatic data transfer Used as needed |
| Clinical Advantages | Out-of-clinic measurementsComparability to Goldmann tonometry (mmHg) | 24-hour pressure profile Regular measurements Patient independent Nocturnal measurements Measurements during real-life activities Data obtained during lens removal appointment |
Out-of-clinic measurements Measurements during real-life activities Wireless data communication through dedicated server Comparability to Goldmann tonometry (mmHg) |
| Patient Disadvantages | Need to master the techniqueNeed to write down results Need to communicate results to the specialist |
Two appointments Inability to drive Visible facial patch Cumbersome recorder |
Surgical implantation |
| Clinical Disadvantages | Reliability dependent on patient training/dexterityFrequency dependent on patient involvement Patient in charge of data communication No continuous measurements Inability to provide measurements during real-life activities or sleep |
Only 24 hours Arbitrary units (mVeq) noncomparable to mmHg No interpretation guidelines available |
Frequency dependent on patient involvement Continuous measurements require additional accessories and may be cumbersome |
| Specific Practicalities and Potential Challenges | Patient trainingPatient motivation Device rental/lending Patient in charge of data Remote data interpretation |
Topography needed Some insurance coverage Specialist lens fitting Impact on patient’s life Requires 2 appointments Data interpretation of arbitrary unit profile |
Simple patient training Patient motivation Often self funded Surgical implantation Remote data interpretation |
| Approvals | CE, FDA | CE, FDA, PMDA | CE |
| CE, European Conformity; FDA, U.S. Food and Drug Administration; PMDA, Japanese Pharmaceuticals and Medical Devices Agency. | |||
The Technology
Home Tonometers
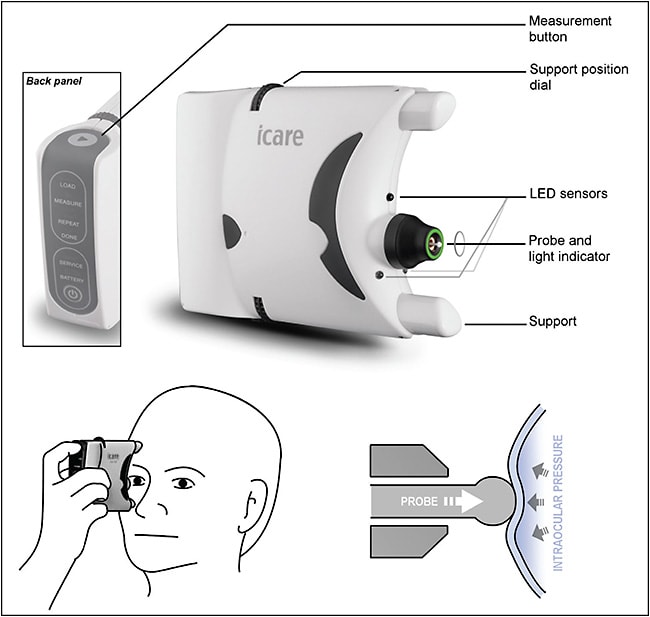
Research into new techniques to measure IOP has naturally seen home tonometry options emerge as simple variations from these technologies. In recent years, rebound tonometry has grown in popularity due to its practicality, relative reliability, and minimal invasiveness, and a self-use option was designed by iCare Finland Oy: the iCare Home (Figure 1).16,17 It consists of a handheld device in which the push of a button propels a small probe toward the patient’s cornea. The speed of deceleration caused by corneal contact is used to determine IOP. The size of the probe and the speed of the measurement makes it appropriate to use without topical anesthesia, and studies have demonstrated good correlation between iCare measurements and GAT.18 However, achieving such levels of reliability requires sound patient training and cooperation, as the usefulness of measurements depends on a patient’s technique (ie, frequency, timing, probe angle, gaze direction, blinking, and targeting central cornea), adding an extra layer of complexity to the interpretation of tonometry results.19 This is especially true because patients who are blinded to their measurements are unable to act upon abnormal results and may gradually lose motivation. Furthermore, while home tonometry clearly gives some degree of control back to patients, this does not come without its own set of challenges. Beyond the issue of patient education and its cost to the practice, self-tonometry poses the more complex questions of data processing practicalities and responsibilities.
Contact Lens Sensors
Despite a first concept designed in 1974 by Green and Gilman, effectively pioneering 24-hour telemetry, it took more than 40 years for the first contact lens sensor (CLS) to become commercially available.20 Produced by the Swiss company Sensimed, the Triggerfish CLS is a disposable soft silicone lens with an embedded microelectromechanical system (Figure 2). The Triggerfish CLS does not actually measure IOP; it measures limbal strains, changes of which are assumed to reflect a composite of IOP, intraocular volume, and biomechanical properties of the eye. Because data represent a strain, they are measured in an arbitrary unit (millivolt equivalents [mVeq]) and cannot be translated into millimeters of mercury.21 A recording session lasts 24 hours, during which the CLS microprocessor continuously transmits an output signal to an adhesive antenna stuck on the patient’s face, which is itself connected to a portable recorder worn at the patient’s waist. The relatively noninvasive nature of the Triggerfish CLS, the truly continuous measurement it provides, and its capacity to detect nocturnal IOP variations make it a versatile tool for the assessment of complicated glaucoma cases, but its day-to-day use would require some adjustment within a clinical practice. Access to a trained contact lens practitioner is crucial to ensuring adequate lens fitting, and patient cooperation with the process is essential, considering the impact the device can have on daily activities, including driving. Additionally, the results, currently provided in mVeq, only provide the outline of a variation profile and are left to individual interpretation.22
A newer pressure-measuring contact lens is currently being tested by Sensimed to provide absolute IOP readings in millimeters of mercury. A recent proof-of-concept study showed that the mean IOP difference between the lens and tonometry on the same eye was within ±5 mmHg in 75% for GAT and 87.5% for dynamic contour tonometry.23
Implantable Sensors
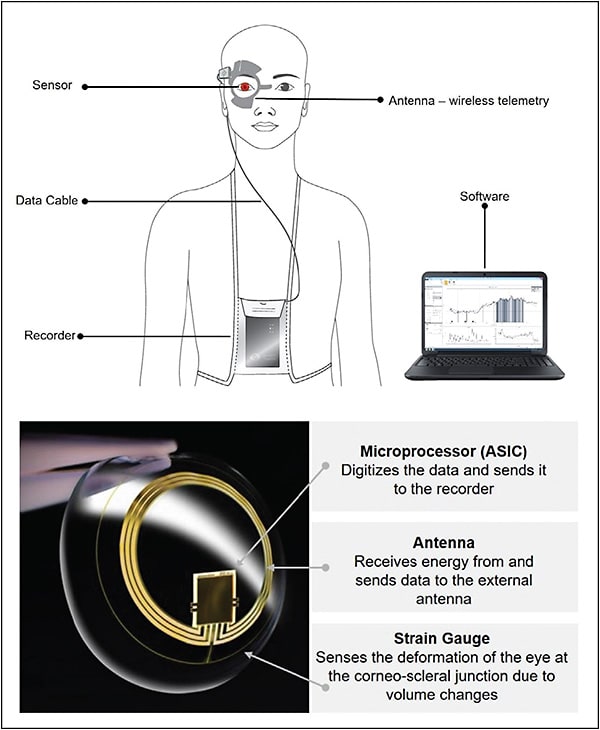
Implandata introduced the first implantable continuous monitoring device: the Eyemate (Figure 3).24 The sensor itself weighs approximately 0.1 g and is implanted in the ciliary sulcus during routine cataract surgery. It is designed to stay in the patient’s eye indefinitely. To obtain an IOP measurement, the patient holds a handheld reader unit (that resembles an old-fashioned TV remote) within 5 cm of their eye and presses a button. This is all the cooperation required from the patient. This activates an electromagnetic coupling sequence between the reader and the sensor, which supplies power to the sensor and converts the measurement into a digital signal that is transmitted to the reader by radiofrequency. Intraocular pressure (IOP) is then displayed on an LED screen on the reader unit and is stored until the data are wirelessly transferred to their ophthalmologist.

The Eyemate has been available in Europe since 2017, and clinical results are promising. Its implantable nature, however, reserves it for patients in more advanced stages of disease. A suprachoroidal version of the sensor is currently being tested for younger or pseudophakic patients. Less of a diagnostic test and more a monitoring tool, it is clear that simple and accurate self-monitoring of IOP has strong potential to improve the care and follow-up of glaucoma patients. As with every self-performed measure, however, the frequency and timing of the measurements will fully depend on the patients’ involvement with their care.22
The Benefits
While a majority of glaucoma patients pose no major diagnostic or therapeutic difficulty, every glaucoma specialist still encounters, with varying degrees of frequency, patients who hover in a gray area and over whom no unanimous consensus can be reached. The lack of understanding of the exact pathophysiological principles underlying glaucoma is most certainly to blame. However, in this uneven battle, ophthalmologists are not equipped with the right weapons to fight the disease. Indeed, if IOP is still the sole modifiable risk factor for glaucoma progression, glaucoma management should not rely on only a vague estimation of its value measured over a couple of seconds out of the 31,536,000 seconds in a year. With such a strong emphasis on IOP, it is no wonder that continuous measurement of IOP is the dream of many glaucoma specialists.
Continuous IOP measurement would significantly reduce the risks of interpersonal variability and lack of repeatability currently observed with some applanation or rebound techniques. Most importantly, it would provide a more subtle and accurate representation of real-life IOP through its intrinsic fluctuations. This specific point may be of particular interest, as there may be more to IOP fluctuations than meets the eye. Recent studies suggest that such variations, including nocturnal pressure spikes, could have a direct impact on glaucoma progression, regardless of absolute IOP values.25,26 Then, in the current economic climate where clinicians are increasingly pressured to optimize their use of health care resources, empowering patients with the ability to control their own IOP out of the clinical setting could allow ophthalmologists to space out appointments while monitoring their patients even more closely. Furthermore, besides freeing up time and financial resources for new and more complex cases, the ability to assess IOP both remotely and accurately provides glaucoma specialists with a very enviable tool in the context of pandemics. Indeed, only rarely in medicine can an objective clinical test be performed without even having to physically see a patient and, what’s more, meaningfully influence their management. This is what IOP telemetry has to offer, in the current COVID-19 era and beyond.
Recently, more reports have come out illustrating how close IOP monitoring could detect nonsymptomatic IOP variations that would otherwise go unnoticed. In a published case report by Rüfer et al, a patient even found a rare connection between the administration of dorzolamide eyedrops and the subsequent IOP increases without any medical input.27 Patient testimonials and anecdotal evidence suggest this is far from an isolated case. Indeed, glaucoma patient groups, such as the FitEyes online community (www.fiteyes.com/eye-pressure-home-monitoring/testimonials ), abound with individual stories of how self-monitoring has allowed some of these highly educated patients to fine-tune their treatments or adapt their daily activities to keep their IOP in range with a precision that far exceeds the scope of the advice given in most, if not all, specialist clinics. This suggests that placing patients in charge and providing them with the means to easily and accurately self-monitor could improve glaucoma care by detecting IOP aberrations earlier and eliciting causative factors with more ease. The increased frequency of IOP measurement associated with the democratization of such sensors is likely to lead to an increased incidence of abnormal pressure readings. Although this may represent crucial information on a patient’s individual IOP control, more research will be needed to determine the clinical relevance of every fluctuation captured through continuous monitoring, and more hindsight will be needed to determine who will be responsible for the assessment of such fluctuations — especially when the democratization of telemetry turns the odd out-of-hour check into a continuous flow of thousands of IOP measurements. While this opens up new areas of liability, we suspect that the abundance of data collected via continuous telemetry is also likely to significantly advance glaucoma research and ultimately pave the way for truly individualized glaucoma care.
Conclusion
Today, 3 very different but equally promising options exist to achieve out-of-clinic IOP monitoring. All of them have proved their safety profile and accuracy, but rather than being rivals, their clinical indications complement one another. In their current form, their use and purpose remain specific and targeted. However, this is likely to change in the upcoming years, with the advent of new, more versatile devices, and the now increasingly likely persistence of postpandemic distancing measures in health care. GP
References
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711-1720.
- Gloor BR. Hans Goldmann (1899-1991). Eur J Ophthalmol. 2010;20(1):1-11.
- Kouchaki B, Hashemi H, Yekta A, Khabazkhoob M. Comparison of current tonometry techniques in measurement of intraocular pressure. J Curr Ophthalmol. 2016;29(2):92-97.
- Kawai M, Kawai N, Nakabayashi S, Kinouchi R, Yoshida A. Comparison of intraocular pressure variability in glaucoma measured by multiple clinicians with those by one clinician. Int Ophthalmol. 2017;37(1):95-101.
- McCafferty S, Lim G, Duncan W, et al. Goldmann tonometer error correcting prism: clinical evaluation. Clin Ophthalmol. 2017;11:835-840.
- Ottobelli L, Fogagnolo P, Frezzotti P, et al. Repeatability and reproducibility of applanation resonance tonometry: a cross-sectional study. BMC Ophthalmol. 2015;15:36.
- Pearce JG, Maddess T. The clinical interpretation of changes in intraocular pressure measurements using Goldmann applanation tonometry: a review. J Glaucoma. 2019;28(4):302-306.
- Mansouri K, Weinreb RN, Medeiros FA. Is 24-hour intraocular pressure monitoring necessary in glaucoma? Semin Ophthalmol. 2013;28(3):157-164.
- Mansouri K, Weinreb RN, Medeiros FA. Correction to review: is 24-hour intraocular pressure monitoring necessary in glaucoma? Semin Ophthalmol. 2014;29(1):56.
- Cheng J, Xiao M, Xu H, et al. Seasonal changes of 24-hour intraocular pressure rhythm in healthy Shanghai population. Medicine (Baltimore). 2016;95(31):e4453.
- Nishino K, Yoshida F, Nitta A, Saito M, Saito K. [Transient elevation of intraocular pressure in primary open-angle glaucoma patients after automated visual field examination in the winter]. Nippon Ganka Gakkai Zasshi. 2013;117(12):990-995.
- Qureshi IA, Xi XR, Khan IH, Wu XD, Huang YB. Monthly measurements of intraocular pressure in normal, ocular hypertensive, and glaucoma male subjects of same age group. Changgeng Yi Xue Za Zhi. 1997;20(3):195-200.
- Malerbi FK, Hatanaka M, Vessani RM, Susanna R Jr. Intraocular pressure variability in patients who reached target intraocular pressure. Br J Ophthalmol. 2005;89(5):540-542.
- Medical Advisory Secretariat. Diurnal tension curves for assessing the development or progression of glaucoma: an evidence-based analysis. Ont Health Technol Assess Ser. 2011;11(2):1-40.
- Hatanaka M, Babic M, Susanna R Jr. Reproducibility of the mean, fluctuation, and IOP peak in the diurnal tension curve. J Glaucoma. 2013;22(5):390-392.
- Stamper RL. A history of intraocular pressure and its measurement. Optom Vis Sci. 2011;88(1):E16-E28.
- Quérat L, Chen E. Clinical use of iCare Home tonometer. Acta Ophthalmol. 2020;98(1):e131-e132.
- Poostchi A, Mitchell R, Nicholas S, et al. The Icare rebound tonometer: comparisons with Goldmann tonometry, and influence of central corneal thickness. Clin Experiment Ophthalmol. 2009 Sep;37:687-691.
- Nakakura S. Icare rebound tonometers: review of their characteristics and ease of use. Clin Ophthalmol. 2018;12:1245-1253.
- Greene ME, Gilman BG. Intraocular pressure measurement with instrumented contact lenses. Invest Ophthalmol. 1974;13(4):299-302.
- De Smedt S, Mermoud A, Schnyder C. 24-hour intraocular pressure fluctuation monitoring using an ocular telemetry Sensor: tolerability and functionality in healthy subjects. J Glaucoma. 2012;21(8):539-544.
- Gillmann K, Bravetti GE, Niegowski JL, et al. Using sensors to estimate intraocular pressure: a review of intraocular pressure telemetry in clinical practice. Exp Rev Ophthalmol. 2019;14(6):263-276.
- Wasilewicz R, Varidel T, Simon-Zoula S, Schlund M, Cerboni S, Mansouri K. First-in-human continuous 24-hour measurement of intraocular pressure and ocular pulsation using a novel contact lens sensor Br J Ophthalmol. 2020. [Online ahead of print]
- Melki S, Todani A, Cherfan G. An implantable intraocular pressure transducer: initial safety outcomes. JAMA Ophthalmol. 2014;132(10):1221-1225.
- De Moraes CG, Jasien JV, Simon-Zoula S, Liebmann JM, Ritch R. Visual field change and 24-hour iop-related profile with a contact lens sensor in treated glaucoma patients. Ophthalmology. 2016;123(4):744-753.
- Gillmann K, Young CC, Stanley J, et al. Relationship between contact lens sensor output parameters and visual field progression in open-angle glaucoma: assessment of a practical tool to guide clinical risk-assessment. J Glaucoma. 2020;29(6):461-466.
- Rüfer F, Gillmann K, Choritz L, Thieme H, Weinreb RN, Mansouri K. The value of intraocular pressure telemetry in monitoring the therapeutic effect of glaucoma medications. J Glaucoma. 2020;29(6):e38-e40.










