Primary congenital glaucoma (PCG) is the most common type of childhood glaucoma and is characterized by high intraocular pressure (IOP), glaucomatous optic nerve head damage, and structural damage secondary to high IOP, including globe enlargement, tears in the Descemet membrane, and corneal edema. Primary congenital glaucoma is mostly genetically determined with mutations at the GLC3A locus located on chromosome 2p21, known as CYP1B1, responsible for the majority of genetic alterations in PCG.1,2 There is a genotype–phenotype correlation in PCG; glaucoma presents earlier and is more severe in PCG cases with CYP1B1 mutations.2,3,4 The presumed pathology is delayed or failed angle genesis secondary to arrested maturation of neural crest cells; however, the exact mechanism is not completely characterized. The classical triad of epiphora, blepharospasm, and photophobia could be present in more than 80% of cases.5 Gonioscopy findings include high and flat insertion of the iris, peripheral iris hypoplasia, and absence of angle recess. However, many of these changes are nonspecific and may be difficult to appreciate.
The ultimate goal of childhood glaucoma management is to provide lifelong vision. Medications alone are not completely effective, because of the anomalous drainage angle, and because medications have serious side effects and high nonresponder and noncompliance rates. Therefore, surgery is the main therapeutic option. Angle-based surgery is the primary surgical procedure for most ophthalmologists.
Enhancing Physiologic Outflow With Angle-based Surgeries
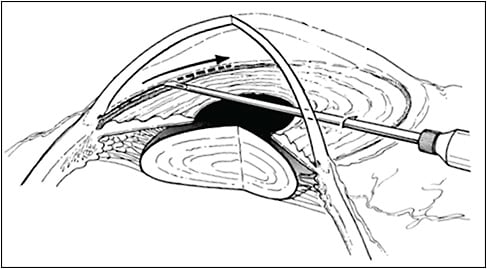
According to a worldwide surgical consensus survey, goniotomy is the most common angle surgery performed for PCG. Goniotomy was the first reported microinvasive glaucoma surgery (MIGS) introduced by Otto Barkan in 1936.6 The procedure only requires a goniolens and goniotomy knife to incise through the trabecular meshwork (TM) and inner wall of Schlemm’s canal (SC; Figure 1). The operating goniolens should leave enough area of the exposed cornea to allow comfortable maneuvering of the knife. A Barkan knife, microvitreoretinal blade, or needle (23 gauge or 25 gauge) could be used to cut through nasal mid-TM for 120° to 180° while the anterior chamber (AC) is maintained with viscoelastic. The incision should be as superficial as possible with no resistance along the incision track. A distinctive white cleft appears in the wake of the incision as elastic scleral spur falls back and pulls down the iris root and posterior lip of the bisected TM. The most frequent postoperative observation is hyphema; however, rarely, inadvertent damage to the iris and lens could happen, especially in the presence of a shallow chamber or for an inexperienced surgeon. Goniotomy enjoys a high success rate ranging from 58% to 95% and remains one of the most widely used surgical methods for PCG.7-10
Trabecular disruption using an ab externo approach with a trabeculotome was first described by Burian.11 A decade later, in 1970, Harms and Dannheim introduced the Harms trabeculotome to cut through the inner wall of SC and the TM and create a limbal scleral flap to better localize SC.12 The Harms trabeculotome has a right- and a left-sided blade to disrupt the trabecular tissue with 2 parallel arms for external guidance. A fornix-based peritomy either in the superior quadrant or temporal quadrant is created. A rectangular half-thickness 3-mm to 4-mm scleral flap is fashioned, followed by exposure of SC through careful radial scleral cutdown at the transition zone until egress of aqueous humor is noted. The trabeculotome is then passed into each end of SC and rotated into the AC.
Finding SC can be challenging in some cases, mainly because of inadequate depth of the incision or second flap. However, it has been reported that SC cannot be identified in 11% to 15% of cases due to distorted limbal anatomy in advanced PCG cases.8
Rigid probe trabeculotomy has an overall success rate of 60% to 85%.5,8,13 Although some studies showed that both goniotomy and ab externo trabeculotomy yield similar results,13-15 others reported a higher success rate with ab externo trabeculotomy.16,17
Several studies have indicated that more extensive opening of the angle results in a higher success rate.5,18,19 Suture-assisted circumferential trabeculotomy was first described by Beck and Lynch in 1995, with a success rate ranging from 52% to 90%.20-22 However, threading a suture into SC entails a risk of false passage and misdirection into the suprachoroidal space and it may be difficult to pass the suture through 360° of SC.
In recent years, an illuminated microcatheter (iTrack; Ellex) has been successfully used to completely cannulate SC. The flexible microcatheter has an illuminated tip that allows visualization of the tip of the microcatheter and is visible through the sclera. In general, the scleral flap is larger in this procedure in comparison to rigid prob trabeculotomy. A 5 mm x 5 mm half-thickness superficial flap is dissected to the peripheral clear cornea. A second flap is created that is slightly smaller than the superficial flap, with a depth that is deep enough for the choroid/ciliary body to become visible underneath few remaining scleral fibers (Figure 2). Careful dissection in this plane toward the limbus will expose and unroof SC. Once SC is identified, a paracentesis can be made to decompress the eye and expand SC. The illuminated microcatheter is then passed into SC while it is kept in an appropriate plane and fed into the canal in small increments. There should be minimal or no resistance along the course of the microcatheter; if the light is not visible, it should be withdrawn until the light is visible and appropriate position is verified. Once the catheter has successfully passed circumferentially, the 2 ends of the microcatheter are grasped with forceps and gently pulled apart. Several studies, including a randomized clinical trial, demonstrated higher success rates with this procedure than with sectoral ab-externo trabeculotomy with the rigid probe.19,23,24

Bypassing TM to Achieve Low IOP in Advanced PCG
The main indication for trabeculectomy in PCG is the failure of angle surgery. However, it could be considered as a first-line procedure, alone or combined with angle surgery, in severe PCG eyes. The success of childhood trabeculectomy is lower compared to adults due to numerous risk factors, including young age, conjunctival scarring secondary to previous surgeries, vigorous wound healing response, and lack of cooperation with postoperative examination or drops. While trabeculectomy can achieve low IOPs with less dependence on medications, there are many challenges for successful trabeculectomy. Bleb-related infection is of particular concern in childhood trabeculectomy, with a reported incidence of endophthalmitis in up to 9% of patients.25 The responsible organisms for infection are consistent with adult trabeculectomy.26 To avoid bleb-related infection, antiscarring agents should be cautiously selected per the patient’s risk factors and administered over the large area, and the scleral flap should be created in such a fashion that a more posterior flow is achieved.27 A large retrospective study showed that higher doses of mitomycin C (MMC) concentrations (0.2 mg/mL or 0.4 mg/mL) and contact times (2 minute to 5 minutes) were not associated with higher success rates.25,28,29 Therefore, while limiting the concentration and time of the exposure may not affect the efficacy of the procedure, it may potentially reduce the complication rates.
Hypotony is another major sight-threatening complication in childhood trabeculectomy, especially in buphthalmic PCG eyes with an incidence comparable to adults.30 Early hypotony is often due to overfiltration and can be avoided if the scleral flap has adequate thickness and is closed tightly to allow no or only minimal leakage. Releasable sutures are preferred, because they can be loosened or removed later in the postoperative period when a strong wound healing response starts to obliterate aqueous drainage through the site of surgery. Overall, the success of MMC childhood trabeculectomy varies from 60% to 90% with short follow-up reports,25,28,29 but success decreases to almost 50% with longer term follow-up.31,32
Since Molteno first reported using glaucoma drainage devices (GDDs) in pediatric glaucoma,33 they have increasingly been used in the management of PCG. The reported success rates for GDDs in PCG are widely variable from 31% to 97%.34-37
Studies that have compared MMC-augmented trabeculectomy with GDD in the management of PCG showed conflicting results.38-40 However, the type of surgery sometimes is dictated by the morphologic features of the eye. Creating a scleral flap of sufficient thickness is impossible in grossly buphthalmic eyes, which makes GDDs the preferred approach.
Glaucoma drainage devices are associated with more complications that need reoperation compared to trabeculectomy.41 Tube-related complications are among the most common complications following GDD implantation in PCG eyes. Significant IOP reduction after shunt procedures results in anterior migration of the tube in elastic PCG eyes and can potentially damage the corneal endothelium.35,36 Some surgeons prefer to insert the tube 1 mm posterior to the limbus and closer to the iris to compensate for possible anterior displacement of the tube. External tube ligation of both valved and nonvalved GDDs has also been described to avoid sight-threatening complications of early hypotony including anterior tube migration. The risk of tube exposure could be reduced by passing the tube through a scleral tunnel 2 mm to 3 mm posterior to the limbus and covering the tube with a full-thickness scleral graft.
Conclusion
Surgical management of PCG has improved significantly over the past few decades, and several surgical approaches have stood the test of time. As of 2020, there is no evidence to support the role of MIGS in the management of PCG; however, MIGS offers theoretical advantages over conventional surgeries and potentially enhances their outcomes. The ideal surgical approach should effectively and safely reduce the IOP and last for the patient’s lifetime. The ultimate goal of PCG management is to provide lifelong vision to affected children. GP
References
- Chakrabarti S, Kaur K, Kaur I, et al. Globally, CYP1B1 mutations in primary congenital glaucoma are strongly structured by geographic and haplotype backgrounds. Invest Ophthalmol Vis Sci. 2006;47(1):43-47.
- Yazdani S, Miraftabi A, Pakravan M, et al. Phenotype and genotype correlation in Iranian primary congenital glaucoma patients. J Glaucoma. 2016;25(1):33-38.
- Stamper RL, Lieberman MF, Drake MV, Becker B. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas. Elsevier Health Sciences, Amsterdam, the Netherlands; 2009.
- Weinreb RG, Papadopoulos M, Grigg J, et al. Childhood glaucoma. World Glaucoma Association Consensus Series 9. Kugler Publications. 2013.
- Esfandiari H, Basith SST, Kurup SP, et al. Long-term surgical outcomes of ab externo trabeculotomy in the management of primary congenital glaucoma. J AAPOS. 2019;23(4):222.e1-e222.e5.
- Barkan O. A new operation for chronic glaucoma: restoration of physiological function by opening Schlemm’s canal under direct magnified vision. Am J Ophthalmol. 1936;19(11):951-966.
- Mendicino ME, Lynch MG, Drack A, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 trabeculotomy versus goniotomy. J AAPOS. 2000;4(4):205-210.
- Al-Hazmi A, Awad A, Zwaan J, Al-Mesfer SA, Al-Jadaan I, Al-Mohammed A. Correlation between surgical success rate and severity of congenital glaucoma. Br J Ophthalmol. 2005;89(4):449-453.
- Papadopoulos M, Cable N, Rahi J, Khaw PT; BIG Eye Study Investigators. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007;48(9):4100-4106.
- Bowman RJC, Dickerson M, Mwende J, Khaw PT. Outcomes of goniotomy for primary congenital glaucoma in East Africa. Ophthalmology. 2011;118(2):236-240.
- Burian HM. A case of Marfan’s syndrome with bilateral glaucoma. With description of a new type of operation for developmental glaucoma (trabeculotomy ab externo). Am J Ophthalmol. 1960;50:1187-1192.
- Harms H, Dannheim R. Epicritical consideration of 300 cases of trabeculotomy “ab externo.” Trans Ophthalmol Soc U K. 1970;89:491-499.
- Chang TC, Cavuoto KM. Surgical management in primary congenital glaucoma: four debates. J Ophthalmol. 2013;2013:612708.
- Papadopoulos M, Edmunds B, Fenerty C, Khaw PT. Childhood glaucoma surgery in the 21st century. Eye. 2014;28(8):931-943.
- Chen TC, Chen PP, Francis BA, et al. Pediatric glaucoma surgery: a report by the American Academy Of Ophthalmology. Ophthalmology. 2014;121(11):2107-2115.
- Zagora SL, Funnell CL, Martin FJ, et al. Primary congenital glaucoma outcomes: lessons from 23 years of follow-up. Am J Ophthalmol. 2015;159(4):788-796.
- McPherson SD, Berry DP. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol. 1983;95(4):427-431.
- Sarkisian SR Jr. An illuminated microcatheter for 360-degree trabeculotomy [corrected] in congenital glaucoma: a retrospective case series. J AAPOS. 2010;14(5):412-416.
- Lim ME, Neely DE, Wang J, Haider KM, Smith HA, Plager DA. Comparison of 360-degree versus traditional trabeculotomy in pediatric glaucoma. J AAPOS. 2015;19(2):145-149.
- Beck AD. 360° Trabeculotomy for primary congenital glaucoma. Archives of Ophthalmology. 1995;113(9):1200-1202.
- Mendicino ME, Lynch MG, Drack A, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J AAPOS. 2000;4(4):205-210.
- Beck AD, Lynn MJ, Crandall J, Mobin-Uddin O. Surgical outcomes with 360-degree suture trabeculotomy in poor-prognosis primary congenital glaucoma and glaucoma associated with congenital anomalies or cataract surgery. J AAPOS. 2011;15(1):54-58.
- Shi Y, Wang H, Yin J, et al. Microcatheter-assisted trabeculotomy versus rigid probe trabeculotomy in childhood glaucoma. Br J Ophthalmol. 2016;100(9):1257-1262.
- Shakrawal J, Bali S, Sidhu T, Verma S, Sihota R, Dada T. Randomized trial on illuminated-microcatheter circumferential trabeculotomy versus conventional trabeculotomy in congenital glaucoma. Am J Ophthalmol. 2017;180:158-164.
- Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology. 2000;107(3):422-429.
- Freedman SF, McCormick K, Cox TA. Mitomycin C-augmented trabeculectomy with postoperative wound modulation in pediatric glaucoma. J AAPOS. 1999;3(2):117-124.
- Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus- versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology. 2003;110(11):2192-2197.
- Susanna R Jr, Oltrogge EW, Carani JC, Nicolela MT. Mitomycin as adjunct chemotherapy with trabeculectomy in congenital and developmental glaucomas. J Glaucoma. 1995;4(3):151-157.
- al-Hazmi A, Zwaan J, Awad A, al-Mesfer S, Mullaney PB, Wheeler DT. Effectiveness and complications of mitomycin C use during pediatric glaucoma surgery. Ophthalmology. 1998;105(10):1915-1920.
- Fulcher T, Chan J, Lanigan B, Bowell R, O’Keefe M. Long-term follow up of primary trabeculectomy for infantile glaucoma. Br J Ophthalmol. 1996;80(6):499-502.
- Jayaram H, Scawn R, Pooley F, et al. Long-term outcomes of trabeculectomy augmented with mitomycin c undertaken within the first 2 years of life. Ophthalmology. 2015;122(11):2216-2222.
- Junior JG, Borges-Giampani AS, Carani JCE, Oltrogge EW, Junior RS. Efficacy and safety of trabeculectomy with mitomycin C for childhood glaucoma: a study of results with long-term follow-up. Clinics. 2008;63(4):421-426.
- Molteno ACB. Children with advanced glaucoma treated by draining implants. SAfr Arch Ophthalmol. 1973;1:55-61.
- Coleman AL, Smyth RJ, Wilson MR, Tam M. Initial clinical experience with the Ahmed Glaucoma Valve implant in pediatric patients. Arch Ophthalmol. 1997;115(2):186-191.
- O’Malley Schotthoefer E, Yanovitch TL, Freedman SF. Aqueous drainage device surgery in refractory pediatric glaucomas: I. Long-term outcomes. J AAPOS. 2008;12(1):33-39.
- Pakravan M, Esfandiari H, Yazdani S, et al. Clinical outcomes of Ahmed glaucoma valve implantation in pediatric glaucoma. Eur J Ophthalmol. 2019;29(1):44-51.
- Razeghinejad MR, Kaffashan S, Nowroozzadeh MH. Results of Ahmed glaucoma valve implantation in primary congenital glaucoma. J AAPOS. 2014;18(6):590-595.
- Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003;136(6):994-1000.
- Hill R, Ohanesian R, Voskanyan L, Malayan A. The Armenian Eye Care Project: surgical outcomes of complicated paediatric glaucoma. Br J Ophthalmol. 2003;87(6):673-676.
- Mokbel TH, El Hefney EM, Hagras SM, et al. Launching a paradigm for first and redo-surgery in primary congenital glaucoma: institutional experience. Int J Ophthalmol. 2019;12(2):226-234.
- Ou Y, Yu F, Law SK, Coleman AL, Caprioli J. Outcomes of Ahmed glaucoma valve implantation in children with primary congenital glaucoma. Arch Ophthalmol. 2009;127(11):1436-1441.









