After decades of relative senescence, the glaucoma surgical landscape has recently exploded with innovation. The initial push was in the microinvasive glaucoma surgical space, with ab-interno approaches that did not create a bleb and emphasized safety over efficacy. The pendulum is now swinging back in the direction of efficacy, and when seeking efficacy, subconjunctival filtration is still king. Thus, innovation in the subconjunctival filtering devices space is what is driving most of the innovation currently. We will take a look at what is currently available.
Subconjunctival filtration devices are basically tubes that shunt aqueous into the subconjunctival space. They can be divided into those that have a large bore tube attached to a plate and those that with a small bore that do not have an associated plate.
Glaucoma Drainage Implants
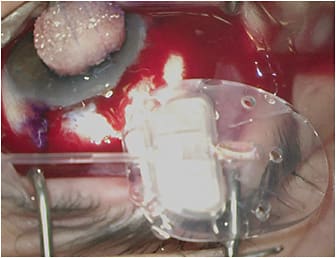
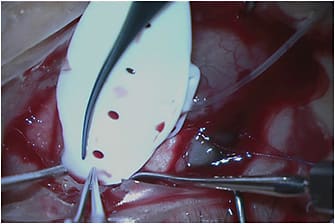
Traditional tube shunts or glaucoma drainage implants (GDIs) are basically large-bore 600-µm (300-µm internal lumen) silicone tubes attached to silicone or polypropylene plates. The silicone tubes are placed in the anterior chamber, ciliary sulcus, or pars plana (in vitrectomized eyes) to drain aqueous into a subconjunctival space maintained by the plate, which is typically secured to the underlying sclera approximately 8 mm to 10 mm posterior to the limbus. The area of the subconjunctival space overlying the plate that collects the aqueous is referred to as a bleb.
The advantage of GDIs over those subconjunctival filtration surgeries that do not utilize a plate (ie, trabeculectomy or Xen 45 [Allergan]) is that the plate of the GDI prevents episcleral fibrosis from obliterating the filtering bleb and thus gives a theoretical survival advantage. In addition, the posterior location leads to healthier, thicker blebs that are much less likely to suffer complications, such as spontaneous leaks and blebitis. The disadvantage of GDIs is that they must be placed far posteriorly on the surface of the sclera (to prevent plate erosion). This region is where Tenon’s capsule is quite thick. Tenon’s capsule serves as a rich source of fibroblasts, increasing the likelihood of aggressive encapsulation of the bleb, which can lead to higher average intraocular pressures (IOPs) and possibly the need for more postoperative medication use than subconjunctival filtration surgeries without a plate, which produce thinner, more anterior blebs. GDI design makes them more prone to several complications: 10% to 12% risk of postoperative diplopia due to proximity of the implant to the extraocular muscles, 2% to 7% risk of erosion of the conjunctiva overlying the tube, and corneal edema as high as 20% due to mechanical trauma and other factors.1-3
GDIs can be categorized by valved and nonvalved devices. Each category has its advantages and disadvantages in various applications.
Valved Glaucoma Drainage Implants
The Ahmed Glaucoma Valve (AGV; New World Medical) is the only widely used GDI that has a valve mechanism. The most commonly used versions of the AGV are the larger 184-mm2-sized FP7 model and the smaller 96-mm2 FP8 model. As the name implies, valved GDIs have a valve or flow restrictor mechanism that decreases the risk of overfiltration and sustained hypotony as a postoperative complication, enabling the surgeon to implant the device and obtain immediate IOP lowering since there is no need to apply a temporary ligature to restrict flow.
Nonvalved Glaucoma Drainage Implants
This group of GDIs includes the Baerveldt Glaucoma Implant (BGI; Johnson & Johnson Vision), the Molteno 3 (Molteno Ophthalmic Ltd.), and the Ahmed ClearPath (ACP; New World Medical). The BGI is available as the BG 103-250 (250-mm2 plate surface area), BG 101-350 (350 mm2), and the Pars Plana 102-350 (350 mm2 with Hoffman elbow for pars plana implantation). The Molteno 3 is available as the SS (185 mm2) and the SL (245 mm2). The ACP comes as the Model 250 (250 mm2) and the Model 350 (350 mm2). The nonvalved GDIs require some form of temporary flow restriction since they have no intrinsic valve mechanism. For most surgeons, this need means completely ligating the tube to prevent aqueous flow in the early postoperative course until a fibrous capsule can form around the plate to limit aqueous flow and prevent hypotony. Most surgeons use an absorbable Vicryl suture for this ligation, which loses its tensile strength at 5 weeks postoperatively and allows for flow through the tube, lowering IOP. This procedure typically works well since it takes approximately 3 to 4 weeks to form a capsule around the plate of the implant to prevent hypotony with tube opening. Early postoperative IOP control before ligature opening can sometimes be problematic if the preoperative IOP is exceedingly high, but the long-term IOP lowering is usually superior to valved GDIs due to the larger surface area size of the plate in nonvalved GDIs.4
Valved vs Nonvalved Glaucoma Drainage Implants
Patients starting with very high IOP, such as those with neovascular glaucoma, will need reliable immediate IOP lowering and thus may not be good candidates for a nonvalved GDI because they may not be able to wait 5 weeks to have the IOP reliably lowered. In such cases, valved GDIs are typically the favored option. In the ABC trial, fewer eyes with neovascular glaucoma went on to a no-light-perception visual outcome in those who received AGV than the BGI, presumably due to the immediate IOP lowering afforded by the AGV.2 Valved GDIs are also often favored in uveitic glaucomas since these patients have very low aqueous production and are very prone to hypotony-related problems when nonvalved GDIs are used.5
Nonvalved GDIs, however, have the advantage of having a much larger surface area than their smaller valved GDI counterparts. The increased surface area on average produces lower IOP over the long term, less postoperative glaucoma medication use, and less surgical failure according to the ABC and AVB trials.4,6 Patients with more advanced disease who require a lower target IOP may be better candidates for nonvalved GDIs.
Subconjunctival Filtration Devices Without a Plate
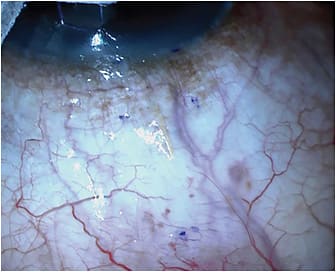
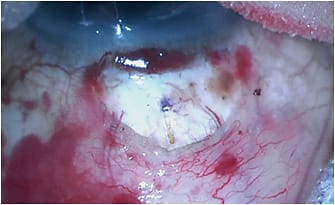
Subconjunctival filtration devices that do not have an associated plate work much more like trabeculectomy surgery than they do GDIs. They produce anterior filtering blebs that are thinner and more diffuse, which are very similar to trabeculectomy blebs. They also require the use of adjunctive antifibrotics, like mitomycin C — also very similar to trabeculectomy. These kinds of blebs are more likely to produce lower IOP with less need for medications if they do not encapsulate or suffer obliteration from episcleral fibrosis. The two devices in this category are the Xen 45 and Preserflo (Santen). Both of these devices are designed to lower the risk of hypotony using a small internal lumen diameter to create enough resistance to aqueous flow to make overfiltration less likely. Preserflo, as of the writing of this article, was still awaiting FDA approval. Our discussion will focus on the Xen 45.
Xen 45
The Xen 45 is currently available in the United States. It is a 6-mm-long tube made of a hydrophilic porcine gelatin crosslinked with glutaraldehyde. It has a 45-µm internal lumen diameter. It is designed around the Hagen-Poiseuille law of laminar flow: the 45-µm internal lumen across a 6-mm length of tubing will produce a resistance to aqueous flow resulting in a 0.02 mL/sec or 1.2 mL/min rate of flow. This rate should produce a 6 mmHg to 8 mmHg flow resistance, which should prevent hypotony.7 When dry, the external diameter is 150 µm, which expands to more than 200 µm when hydrated. This expansion helps to fill the 27-gauge needle tract through which it is delivered and prevent peritubular flow of aqueous. The hydrating process also causes the tube to become soft and flexible, which should decrease the likelihood of erosion through the conjunctiva.
The FDA-approved method of delivery is ab interno, which requires a clear corneal incision and ophthalmic viscoelastic device (OVD) injection to stabilize the anterior chamber. In the opinion of some, this approach leads to a high rate of entanglement within Tenon’s capsule, leading to high rates of bleb needling (32% to 53%).7 Some have advocated using pneumodissection to separate the conjunctiva from the underlying Tenon’s capsule, followed by viscoexpansion of the pneumodissected space with the OVD to create a pocket to deliver the Xen so that is “supra-Tenon’s” and truly subconjunctival. Advocates of this approach tout lower bleb needling rates than with the traditional ab-interno technique.8
It has become very popular recently to deliver the Xen 45 ab externo, but this procedure is off label from its FDA approval. There are some significant advantages to this mode of delivery. The ab-interno approach can only be performed through a temporal clear corneal incision, forcing the surgeon to only be able to place it superonasally. Ab-externo delivery allows the surgeon to place it in any quadrant he or she chooses. The ab-externo approach does not require a corneal incision or OVD injection into the anterior chamber. One group showed in an animal model that ab-externo implantation resulted in a greater aqueous flow rate than an ab-interno approach.9 From a mechanistic perspective, ab-interno delivery forces the needle to pierce through multilayered tissue, like Tenon’s capsule, without the benefit of any countertraction. It makes it very difficult to get through Tenon’s capsule fully without piercing the conjunctiva itself. Going ab externo solves this problem by either allowing the surgeon to easily pierce through Tenon’s capsule by compressing it against the sclera in a transconjunctival approach or mechanically clearing it away with scissors in an open conjunctival approach.
Ab-externo delivery can be performed through either a closed conjunctiva (transconjunctival) or an open conjunctiva via a peritomy. In the ab-externo transconjunctival approach, the 27-gauge needle tip of the Xen 45 injector is used to pierce through the conjunctiva 7-10 mm posterior to the limbus and advance through the subconjunctival space. Then, it pierces through the sclera 2 mm to 2.5 mm posterior to the limbus at a shallow 15° angle to enter the anterior chamber just anterior to the trabecular meshwork and then deploy the Xen. The Xen can then be adjusted through the conjunctiva with toothless conjunctival forceps if necessary. This technique benefits from a corneal traction suture or other means to dramatically infraduct the eye, which appears to be a noninferior technique to traditional ab-interno approaches, while lowering the needling rate.10
Ab-externo Xen through an open conjunctiva is the final approach. In a way, one could argue that this approach violates the “minimally invasive” concept of the Xen. However, its advocates tout a much lower needling rate than with the traditional ab-interno approach. The disadvantages of opening the conjunctiva, having to apply cautery, and then closing the conjunctiva are offset by an unobstructed view of the device entry into the sclera and the freedom to easily manipulate the Xen after implantation for optimal placement. Mechanically clearing out a space for aqueous to accumulate under Tenon’s capsule and preventing entanglement of the stent within Tenon’s capsule are major advantages to this approach.11
Summary
The number of choices in the glaucoma surgical armamentarium is continuing its rapid expansion and has now found its way back into the subconjunctival space. With Xen 45, surgeons now have the choice of using a small-bore device that dramatically reduces the risk for hypotony while producing a bleb and IOP characteristics similar to a trabeculectomy. For eyes at greater risk of trabeculectomy or Xen 45 failure, surgeons have more options than ever in the traditional large-bore tube shunt or GDI space. For immediate IOP lowering and greater protection against hypotony, the AGV is the GDI of choice. For potentially greater long-term IOP lowering when immediate IOP reduction is less important, the BGI, Molteno, and ACP are available. Glaucoma is a lifelong disease with no cure, and the more tools that we have at our disposal, the better. GP
References
- Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.e1. doi:10.1016/j.ajo.2011.10.024
- Budenz DL, Feuer WJ, Barton K, et al. Postoperative complications in the Ahmed Baerveldt comparison study during five years of follow-up. Am J Ophthalmol. 2016;163:75-82.e3. doi:10.1016/j.ajo.2015.11.023
- Chaku M, Netland PA, Ishida K, Rhee DJ. Risk factors for tube exposure as a late complication of glaucoma drainage implant surgery. Clin Ophthalmol. 2016;10:547-553. doi:10.2147/OPTH.S104029
- Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308-316. doi:10.1016/j.ophtha.2014.08.043
- Sinha SS, Ganjei AY, McWatters Z, et al. Ahmed versus Baerveldt glaucoma drainage device in uveitic glaucoma: a retrospective comparative study. J Glaucoma. 2020;29(9):750-755. doi:10.1097/IJG.0000000000001583
- Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed versus Baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093-2102. doi:10.1016/j.ophtha.2016.06.035
- Fea AM, Durr GM, Marolo P, et al. XEN® gel stent: a comprehensive review on its use as a treatment option for refractory glaucoma. Clin Ophthalmol. 2020;14:1805-1832. doi:10.2147/OPTH.S178348
- Vera V, Gagne S, Myers JS, Ahmed IK. Surgical approaches for implanting Xen gel stent without conjunctival dissection. Clin Ophthalmol. 2020;14:2361-2371. doi:10.2147/OPTH.S265695
- Lee RM, Bouremel Y, Eames I, Brocchini S, Khaw PT. The implications of an ab interno versus ab externo surgical approach on outflow resistance of a subconjunctival drainage device for intraocular pressure control. Transl Vis Sci Technol. 2019;8(3):58. doi:10.1167/tvst.8.3.58
- Ucar F, Cetinkaya S. Xen implantation in patients with primary open-angle glaucoma: comparison of two different techniques. Int Ophthalmol. 2020;40(10):2487-2494. doi:10.1007/s10792-020-01427-z
- Panarelli JF, Yan DB, Francis B, Craven ER. XEN gel stent open conjunctiva technique: a practical approach paper. Adv Ther. 2020;37(5):2538-2549. doi:10.1007/s12325-020-01278-1









