The glaucoma drainage device (GDD) has been a mainstay for glaucoma surgical care since the introduction of the Molteno device nearly 50 years ago. In spite of the expansion of minimally invasive glaucoma surgery (MIGS), such as the Xen gel stent (Allergan), to include safer subconjunctival filtration, the GDD (or “tube shunt”) remains crucial in the surgical armamentarium of the glaucoma specialist. This article will review the history and development of the GDD, as well as the author’s experience with a new GDD model.
Medical claims data and surveys of glaucoma surgeons demonstrate that GDDs are being selected increasingly over trabeculectomy.1 In a recent analysis of the US Medicare database, the number of aqueous shunt implantations performed annually has risen from 2,356 in 1994 to 12,021 in 2012.2 The Tube Versus Trabeculectomy (TVT) study demonstrated superior results for GDDs vs trabeculectomy in patients with prior trabeculectomy and/or cataract surgery.3 More recently, the Primary Tube versus Trabeculectomy study demonstrated equal rates of failure between both groups.4
Glaucoma drainage devices were first proposed and investigated by Dr. Anthony Molteno in 1969. He had observed that blebs formed by filtration surgery tended to shrink, and he subsequently hypothesized that filtration failure was due to fibrosis and collapse of the bleb. Dr. Molteno theorized that a subconjunctival implanted device with a tube inserted into the anterior chamber would cause the formation of a large capsule. He initially proposed removal of the implant once the capsule was formed, leaving a thick capsule that would not collapse. In a subsequent publication, he transitioned to leaving the implant in place, and the GDD was born.5,6
A variety of plate sizes have been used over the years by different manufacturers, with the original Molteno plate at 133 mm2 and later a double-plate, 2-quadrant design to double the surface area. Today the most popular GDDs are the Ahmed FP7 (New World Medical), the Baerveldt Glaucoma Implant (BGI; Johnson & Johnson Vision), and the Molteno3 (Nova Eye Medical). The sizes are 184 mm2 for the Ahmed FP7, 250 mm2 for the BGI 250, and 350 mm2 for the BGI 350. The Molteno3 is available in 185 mm2 and 245 mm2 models. The size of the plate may influence intraocular pressure (IOP) control, with larger plate area associated with greater IOP lowering, but with increased hypotony-related complications.7 However, there may be an upper limit to plate size and IOP reduction, with the BGI 350 having greater long-term success than the larger (and no longer available) BGI 500.8 Additionally, a retrospective review comparing the BGI 350 to the BGI 250 found no differences in surgical success or complication rates between the 2 plate sizes, perhaps suggesting a broader “sweet spot” in plate size between surgical success and complication risks.9
Although different materials have been used for the outflow plate over the years, today, GDDs consist of 2 different materials: polypropylene, as used in the Molteno3, and silicone, as used in current Baerveldt and Ahmed devices. In clinical studies comparing the older model polypropylene with the current silicone Ahmed GDD, lower IOP levels with fewer supplemental glaucoma medications and longer survival were found with the silicone version.10,11 However, other studies have found similar success among nonvalved GDDs with different plate materials.12 Regardless of plate material and size, the tube that extends from the implant is similar in all current GDDs. This tube is made of silicone with an inner lumen of approximately 0.3 mm and an outer diameter of approximately 0.64 mm.
The most significant difference between the various GDDs is between nonvalved and valved devices. Valved GDDs were developed over the years to address the problem of early postoperative hypotony before the encapsulated bleb forms over the implant plate. Because the valve prevents excess aqueous outflow, valved GDDs have lower rates of hypotony and fewer hypotony-related postoperative complications.13 Valved GDDs also provide for immediate IOP reduction. Nonvalved GDDs require somewhat more surgical preparation and postoperative care. In the Ahmed Baerveldt Comparison (ABC) Study of valved vs nonvalved devices, at 5 years, the BGI group had an average IOP 2 mmHg lower than the Ahmed Glaucoma Valve (AGV) group. Although there were fewer medications in the BGI group on average at most time points, there was no difference between the 2 groups at 5 years. Failures in the 2 groups occurred for different reasons. The AGV group had more failures due to high IOP endpoints (persistently elevated IOP and reoperation for IOP elevation), whereas the BGI group failed due to safety endpoints such as persistent hypotony or loss of light perception vision.14 The Ahmed vs Baerveldt study had a similar design and findings.13
Although there may be some differences in outcomes between various GDDs in use today, often the choice of GDD type is dependent on surgeon preference and patient demographics. In cases of acute neovascular glaucoma with poorer visual potential, in cases of active uveitic glaucoma, and in patients with advanced age, a surgeon might opt for a valved tube (Ahmed FP7) to allow more immediate IOP reduction and to avoid hypotony-related complications. However, in most patients with moderate to advanced primary open-angle glaucoma, secondary open-angle glaucoma, or chronic angle closure glaucoma who have a failed trabeculectomy, a nonvalved GDD is the preferred option for many surgeons.
New World Medical released the nonvalved Ahmed Clearpath GDDs in 250 mm2 (CP250) and 350 mm2 (CP350) sizes in August 2019 (Figure 1). The Clearpath can be useful in those situations where a nonvalved GDD is desired. Features include more anteriorly located suture fixation eyelets and a more flexible plate with a contour that closely conforms to the shape of the eye. The CP250 is a single-quadrant design that fits between the rectus muscles. The CP350 fits securely under the rectus muscles with easy insertion due to a flexible plate design that better conforms to the shape of the globe.
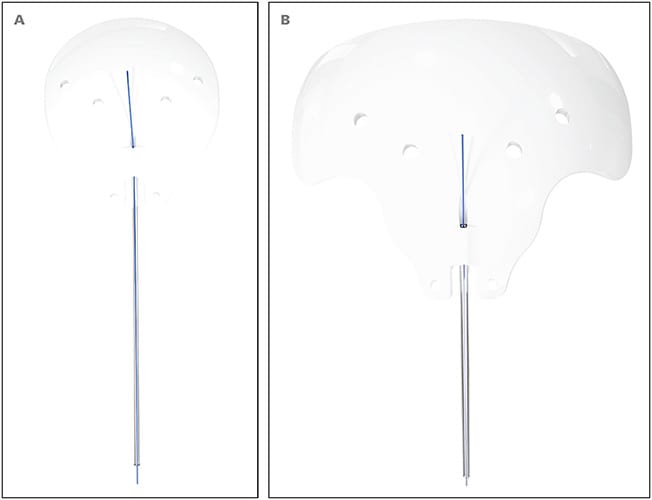
Personal Experience With Implantation
A 350 mm2 GDD can be a useful choice, except in patients with more conjunctival scarring or subjectively higher risk for hypotony, for whom a 250 mm2 device is likely preferred. The Ahmed Clearpath devices come preloaded with a 4-0 polypropolene stent suture. I completely ligate the tube with a 8-0 polyglactin suture. To provide early postoperative IOP lowering, I utilize the 6.5-mm spatulated needle from my 7-0 polyglactin corneal traction suture to fenestrate the tube anterior to the ligature suture. I find that 3 passes completely through the tube is usually sufficient for reasonable IOP control for the first several weeks, with aqueous suppressants often started after the first postoperative week. Although I may remove the stent suture after 1 month in cases with elevated IOP, in most cases, I wait for the ligature suture to dissolve and aqueous flow to egress over the plate at approximately 6 weeks postoperatively. For surgeons who do not utilize a stent suture but desire more IOP lowering before the sixth postoperative week, it is possible to use an argon laser to lyse the ligature after postoperative week 4.
My preferred GDD implant location is in the superotemporal quadrant, although GDD location is often determined by scarring from previous surgeries. If possible, I avoid primary superonasal tube placement due to the superior oblique muscle complex and concern for development of secondary strabismus (pseudo-Brown syndrome).15
My experience with the Clearpath GDDs has been positive. I have found insertion to be similar between the BGI and Clearpath 350 mm2 GDDs, while the Clearpath 250 is easier to implant than the BGI 250 because the narrower plate on the Clearpath 250 allows easy placement between the rectus muscles. I have been able to use a smaller conjunctival incision due to its flexible plate design and anteriorly placed fixation eyelets. Additionally, the anteriorly placed fixation eyelets increase the margin of safety for scleral suture passes. Whereas previous GDD designs require scleral suture fixation at approximately 9 mm from the limbus, the Clearpath allows scleral passes 6 mm from the limbus, lessening the risk of posterior retinal perforation. I especially appreciate this change when operating with residents or fellows.
In cases of failure to control IOP after a first GDD is implanted, a second GDD may be required. The location choice is dependent on what type of GDD was initially used. A 350 mm2 GDD occupies space underneath 2 rectus muscles, so placing a second 350 mm2 GDD requires using the contralateral quadrant. My preference is to place a second GDD in the inferonasal quadrant, and in these situations I usually prefer a nonvalved to a valved GDD due to superior IOP control with a nonvalved GDD. The Clearpath 250 fits nicely between the rectus muscles and has become my preferred second or even third GDD. Its thinner profile allows placement between the rectus muscles, thus enabling placement in an adjacent quadrant in the case of a third GDD implantation.
In a retrospective review we conducted of my recent GDD surgeries, 22 eyes of 22 patients had received the Clearpath (11 CP250 and 11 CP350) and 20 eyes of 20 patients had received the BGI (12 BGI 250 and 10 BGI 350). At 6 months, IOP was 14.8±5.2 mmHg for all BGI cases and 10.3±4.2 mmHg for all Clearpath cases (P=.02). A similar number of medications was found between the 2 groups, and no differences in complication or failure rates were seen. It should be noted that this study was limited by small study size and short follow-up time and these early results do not suggest a difference in efficacy between the BGI and Clearpath.16
Conclusion
There have been many recent advances in glaucoma surgery represented by a burgeoning variety of MIGS procedures. However, some patients require more significant IOP reduction and thus innovation in more traditional glaucoma filtering surgery is needed. The Ahmed Clearpath devices are a welcome addition to the GDD family. GP
References
- Gedde SJ, Kornmann HL. Glaucoma surgery in pseudophakic eyes: Tube shunt first. Surv Ophthalmol. 2017;62(1):108-112. doi:10.1016/j.survophthal.2016.05.003
- Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122(8):1615-1624. doi:10.1016/j.ophtha.2015.04.015
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; Tube Versus Trabeculectomy Study Group. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol. 2009;148(5):670-684. doi:10.1016/j.ajo.2009.06.018
- Gedde SJ, Feuer WJ, Lim KS, et al; Primary Tube Versus Trabeculectomy Study Group. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 3 years of follow-up. Ophthalmology. 2020;127(3):333-345. doi:10.1016/j.ophtha.2019.10.002
- Molteno AC. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol. 1969;53(9):606-615. doi:10.1136/bjo.53.9.606
- Molteno AC. A new implant for glaucoma. Effect of removing implants. Br J Ophthalmol. 1971;55(1):28-37. doi:10.1136/bjo.55.1.28
- Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99(10):1512-1519. doi:10.1016/s0161-6420(92)31772-5
- Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better?. Ophthalmology. 1999;106(12):2312-2318. doi:10.1016/S0161-6420(99)90532-8
- Allan EJ, Khaimi MA, Jones JM, Ding K, Skuta GL. Long-term efficacy of the Baerveldt 250 mm2 compared with the Baerveldt 350 mm2 implant. Ophthalmology. 2015;122(3):486-493. doi:10.1016/j.ophtha.2014.09.002
- Ishida K, Netland PA, Costa VP, Shiroma L, Khan B, Ahmed II. Comparison of polypropylene and silicone Ahmed Glaucoma Valves. Ophthalmology. 2006;113(8):1320-1326. doi:10.1016/j.ophtha.2006.04.020
- Khan AO, Almobarak FA. Comparison of polypropylene and silicone Ahmed valve survival 2 years following implantation in the first 2 years of life [published correction appears in Br J Ophthalmol. 2013 Oct;97(10):1362. Almobarak, F A [corrected to Al-Mobarak, F]]. Br J Ophthalmol. 2009;93(6):791-794. doi:10.1136/bjo.2008.151258
- Smith MF, Doyle JW, Sherwood MB. Comparison of the Baerveldt glaucoma implant with the double-plate Molteno drainage implant. Arch Ophthalmol. 1995;113(4):444-447. doi:10.1001/archopht.1995.01100040060027
- Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed Versus Baerveldt Study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093-2102. doi:10.1016/j.ophtha.2016.06.035
- Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308-316. doi:10.1016/j.ophtha.2014.08.043
- Smith SL, Starita RJ, Fellman RL, Lynn JR. Early clinical experience with the Baerveldt 350-mm2 glaucoma implant and associated extraocular muscle imbalance. Ophthalmology. 1993;100(6):914-918. doi:10.1016/s0161-6420(93)31554-x
- Petkovsek DS. Baerveldt vs. ClearPath tube shunt: six-month outcomes. Poster presented at the American Glaucoma Society 2021 virtual meeting, March 4-7, 2021.









