It has been widely documented that the number of topical glaucoma medications a patient takes can cause chronic subclinical inflammation on the ocular surface.1 This chronic inflammation can cause overexpression of highly immunologically active tissues and have a harmful effect on conjunctival and corneal barrier functions. In addition, due to the nature of the disease itself, inflammatory changes may already be present in varying degrees along the eye structure in patients with glaucoma. Therefore, ocular surface disease (OSD) is extremely common in patients with glaucoma.2
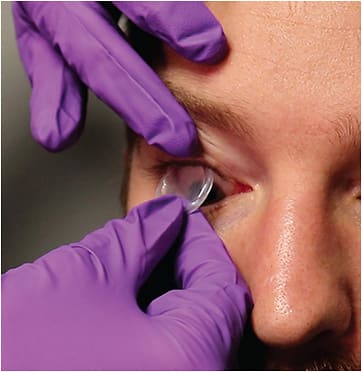
A promising therapy for the treatment of glaucoma-related OSD is cryopreserved amniotic membrane (CAM), a biologic corneal bandage that goes beyond symptom management due to its natural anti-inflammatory and antiscarring properties.3 Prokera (Biotissue) is the only FDA-cleared CAM therapeutic product that provides quick symptom relief and reduces inflammation associated with OSD to help restore the cornea to a normal, healthy state (Figure 1).4
Prevalence of Ocular Surface Disease in Patients With Glaucoma
From personal experience, I have found glaucoma-related OSD to be extremely prevalent. One out of every 2 patients with glaucoma experiences OSD. In the past, glaucoma specialists were only able to rely on patient-reported symptoms, but by the time patients report symptoms, it may be too late because they may have years of chronic inflammation and possible damage.
Glaucoma specialists have traditionally been behind the curve when it comes to dry eye management. Now, we can use imaging modalities to stay ahead of the condition by detecting early disease, even when patients are asymptomatic. For years, glaucoma specialists had the advantage of quantifying dry eye issues. If we see early signs of inflammation on exam, we can perform diagnostics like meibomian gland imaging with Lipiscan (Tearscience) and osmolarity testing with the Tearlab Osmolarity System (Tearlab). Once OSD is further along, the common symptoms patients present with are not just eye redness or foreign body sensation but also unexplainable blurry or fluctuating vision.
The problem of OSD in patients with glaucoma is recognized by most clinicians, but whether it is prioritized is a completely different matter. In the past, glaucoma specialists did not prioritize dry eye; the focus was on preserving the optic nerve and the patient’s sight. However, saving patients from one problem can create another. A patient’s quality of life is intimately connected with their compliance with glaucoma medications, and glaucoma specialists must consider the whole patient with glaucoma.
There are 2 main reasons that glaucoma patients have dry eye. Firstly, studies have shown that benzalkonium chloride does significant damage over time, especially to meibomian glands and the ocular surface. Secondly, the toxic effects of some of the drug molecules in medications used to treat glaucoma can play a role. All of this is additive, so the more medications that a patient takes, or the longer they’ve been on the medications, the worse their OSD is going to be.
Managing Ocular Surface Disease in Patients With Glaucoma
Our practice has a comprehensive dry eye center, and we deal with dry eye in all of its presentations. We pay as much attention to meibomian gland dysfunction (MGD) as we do to the health of the corneal surface. We look at inflammation levels, corneal staining, tear breakup time, osmolarity testing, and meibomian gland imaging in every patient with glaucoma.
For meibomian gland health, we stress a patient’s lid hygiene at home. We use options such as thermal pulsation therapy (Lipiflow; Johnson & Johnson Vision), intense pulsed light (IPL) therapy (Optima IPL; Lumenis) and localized heat therapy (Tearcare; Sight Sciences) for treatment in the office, and we use these modalities in combination, depending on the degree of MGD a patient has. We also find them to be additive. Both Lipiflow and IPL are now FDA approved for the treatment of dry eye.
For corneal health, if the OSD and staining are mild, treatment options such as lubricating and gel eye drops work. For patients with more severe or persistent symptoms, there is cyclosporine, serum tears, and CAM.
Benefits of Cryopreserved Amniotic Membrane Therapy
Cryopreserved amniotic membrane is a great option for healing the cornea because it can improve the quality of life of many patients with glaucoma-related OSD. Traditionally, CAM was used to heal bad burns of the eyes or pterygium surgery, but CAM is also FDA cleared for punctate keratitis.
Patients who have been on multiple medications for many years, whose corneas are badly beaten up or have an extreme form of keratitis, are great candidates for CAM. Cryopreserved amniotic membrane is for patients who have a severe eye drop load and significant keratitis. However, glaucoma specialists should not wait for cases to become so severe. Patients who have any amount of staining that isn’t going away with conservative measures are potential candidates for CAM. The earlier glaucoma specialists can intervene — especially when they see staining — the better, because it is much more difficult to turn around severe dry eye.
Glaucoma specialists will anecdotally reprt that their patients do not use their eye drops because of how severely they affect their eyes. I’ve found that using CAM enables patients to tolerate eye drops for longer periods and therefore makes patients more compliant. Also, for patients with significant staining, CAM can help improve their vision. These findings are not just from personal experience but also from research. A study of 8 patients with glaucoma and punctate keratitis that I presented at the 2019 American Society of Cataract and Refractive Surgery annual meeting found that CAM improved ocular surface integrity in terms of corneal staining, decreased patients’ discomfort, and in some cases increased compliance to their antiglaucoma medication.5 We also showed that some of our patients had improvement in their vision. Since these findings, we have actively offered CAM to our glaucoma patients suffering from punctate keratitis to help improve their quality of life.
One advantage of CAM is that it is cryopreserved, and therefore, it retains growth factors that are specific to live tissues but missing from dehydrated amniotic membranes. The scientists who pioneered live amniotic membrane treatment have shown the healing capacities for the cornea of these growth factors in their studies.3,4
As a live product, CAM can also show longer lasting effects. In my own work, I have found that patients who are treated with CAM continue to feel subjectively better for up 6 months.5 Cryopreserved amniotic membrane also has a heavy-chain hyaluronic acid (HC-HA) and pentraxin 3 (PTX-3) complex that helps to control inflammation and increase wound healing, which isn’t in the dehydrated material.3,4
Implementing Cryopreserved Amniotic Membrane Into Practice
All my conversations with patients about treating their dry eye with CAM are based on their clinical presentation and complaints. I make it a point to tell them that the presence of dry eye, staining, and keratitis is just as important as glaucoma. Should it worsen, my concern is that their dry eye will affect their vision, their eyes will be uncomfortable, and they might not be able to tolerate their therapy long-term. I also tell patients that CAM has healing properties, and it’s not anything they have to manage on their own. Our team will guide them through the process so that it is seamless, and if they have any issues, they have a person to contact at any time.
Inserting CAM into a patient’s eye requires training. Our practice employs a dry eye coordinator who runs our dry eye clinic and oversees these therapies. We train staff properly and set realistic expectations for patients, which all makes a difference.
I haven’t found any interference using CAM alongside other medical or surgical treatments for glaucoma. Patients receive CAM on the eye for 5 days at a time, one eye at a time. Patients continue on their glaucoma drops at the same time because we’re not completely taping the eye shut. This can potentially increase patient compliance over time because their dry eye is getting better, so they are more likely to use their glaucoma medications.
My advice for glaucoma specialists is that they prioritize OSD. In the long run, treatment of OSD makes a huge difference in the compliance and quality of life of patients, especially when glaucoma specialists are expected to treat them for the rest of their lives and are on this journey with them.
When it comes to CAM, the growth factors create nerve regeneration and anti-inflammatory effects that are worth it. Glaucoma specialists should be proactive about offering CAM when indicated. With properly trained staff and attention directed to OSD, patients should be able to go through the process without a lot of concern, and they should experience significant improvement in their dry eye and quality of life as a result. GP
References
- Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11-18. doi:10.1097/ICL.0000000000000544
- Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog Retin Eye Res. 2021;83:100916. doi:10.1016/j.preteyeres.2020.100916
- John T, Tighe S, Sheha H, et al. Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease. J Ophthalmol. 2017;2017:6404918. doi:10.1155/2017/6404918
- McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677-681. doi:10.2147/OPTH.S162203
- Vendal Z, El Sheha H, Tighe S. Management of glaucoma-induced dry-eye disease. Presented at the ASCRS ASOA Annual Meeting; May 4, 2019: San Diego, CA. Accessed July 19, 2022. https://ascrs.confex.com/ascrs/19am/meetingapp.cgi/Paper/57575









