Accurately measuring intraocular pressure (IOP) is crucial for the management of glaucoma. It is widely recognized that IOP is the most important risk factor for the development and progression of primary open-angle glaucoma, and it remains the only modifiable risk factor for this potentially blinding disease.1 Although Goldmann applanation tonometry (GAT) is the gold standard for measuring IOP, this method is limited by its nonportability as well as the requirements of topical anesthesia and skilled personnel for accurate measurements, which restricts its application to professional health care settings.
New approaches to tonometry have been proposed to overcome some of the limitations of GAT. These include alternatives to reduce the influence of corneal thickness, such as the Pascal dynamic contour tonometer (DCT; Ziemer Ophthalmic Systems), or corneal biomechanics, like the Ocular Response Analyzer (ORA; Reichert Technologies), in the IOP measurements. Tonometers have also become more portable, with handheld devices such as the Tonopen (Reichert Technologies) and different versions of iCare devices in the market for years. There are also several air-puff tonometers commercially available that can quickly measure IOP with no need for topical anesthesia and no direct contact with the eye. This landscape continues to evolve. The following update focuses on new devices recently approved or in the pipeline for future approval in the United States, promising innovative solutions to evaluate IOP.
Triggerfish
The Triggerfish Sensor (Sensimed) is currently the only commercially available device for temporary continuous 24-hour IOP monitoring in the United States. Because most decisions concerning glaucoma management are based on single IOP measurements during office visits, the device offers an opportunity to evaluate ocular volume changes that may reflect IOP fluctuations during the day and night. It consists of a soft disposable silicone contact lens with a microsensor that captures spontaneous circumferential changes at the corneoscleral area (Figure 1). It is believed that these small modifications in the curvature of the cornea are due to variations of IOP.2 The sensor transmits a signal to an external antenna located around the patient’s eye and then to a portable recorder, for a total of 288 data points over a 24-hour period. Clinical trials have indicated that the contact lens is well tolerated, with its parameters demonstrating fair to good reproducibility3 and association with the rate of visual field progression in glaucomatous eyes.4 However, the device does not measure pressure, but electric signals owing to volumetric change. Because there is no direct correlation between corneal changes (measured in millivolt equivalent) and IOP (in mmHg), the interpretability of the results is not easily translatable to clinical practice. The cost of the equipment is also a limiting factor.

Eyemate-IO
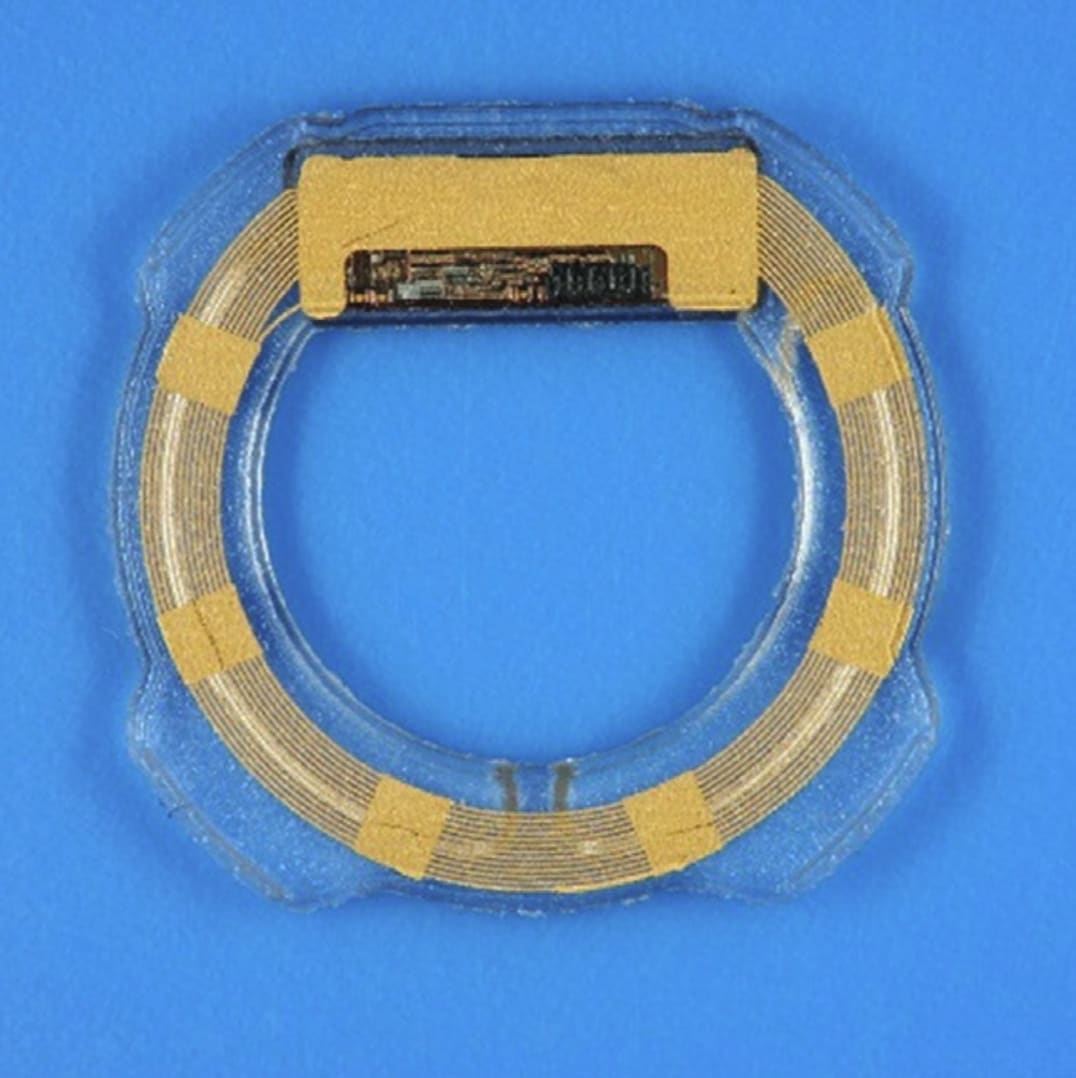
The Eyemate-IO (Implandata Ophthalmic Products) is an implantable ring-shaped device that consists of a microelectromechanical system with pressure and temperature sensors, encapsulated in a silicone ring (Figure 2).5 The device has received CE certification in Europe and it is intended for permanent implantation in the ciliary sulcus. It accompanies an external handheld reader that enables wireless measurements of IOP and wireless power supply. The reader contains a separate pressure sensor that measures the external atmospheric pressure — the difference between the external pressure and the pressure measured by the intraocular sensor represents the IOP, which is displayed on a LED display. The most recent version of the Eyemate-IO can be implanted during cataract surgery in the ciliary sulcus using an injector, and measures up to 0.9 mm in thickness, with 3 available diameters (11.3 mm, 11.7 mm, or 12.1 mm) that can be chosen depending on the size of the eye. A recent open-label single-arm clinical investigation6 in 22 eyes demonstrated marked improvement in the amount of pigment dispersion and transillumination defects induced by the sensor with the second-generation design compared with the first-generation design. Although the Eyemate-IO IOP measurements were, on average, higher than the GAT IOP, the device offers the possibility to be recalibrated noninvasively during extended follow-up.
iCare Home2
The iCare Home (Icare Finland Oy) was the first FDA-approved device for self-measurement of IOP, enabling patients to measure their IOP multiple times at home (Figure 3). It uses the principle of rebound tonometry, estimating IOP by assessing the motion of a probe propelled against the cornea. The duration of the probe's deceleration after touching the cornea is correlated with IOP and translated to an IOP value, with a quicker rebound indicating higher IOP levels. Rebound tonometers are increasingly popular due to their portability and no requirement of topical anesthesia, and have been demonstrated to correlate well to IOP measured with GAT for eyes within a limited range of central cornea thicknesses.7 However, studies have shown that up to 27% of patients were not able to be successfully trained to use the iCare Home,8 which could be partially due to the lack of a live feedback to the patient for optimal positioning of the device. In 2022, a new version received FDA 510(k) clearance, the iCare Home2, with significant improvements to the patient interface. The iCare Home2 features a red ring around the probe that becomes green upon proper alignment.9 It also displays the average of the IOP measurements in a screen in the device and connects to a computer or mobile app, facilitating the storage and analysis of results. The new version of iCare also enables measurements of IOP in different positions, including patients restricted to bed, in a supine position.

Tono-Vera
The Tono-Vera (Reichert Technologies) is a new handheld tonometer that uses the rebound tonometry principle with substantial enhancements in the user interface (Figure 4). These include a proprietary camera-based positioning system that combines a color view of the eye with interactive prompts that ensure proper angulation, distance, and centration, reducing operator-dependent measurement errors. Once the adequate positioning is confirmed, the device automatically acquires IOP measurements using biocompatible probes that quickly touch the cornea. The FDA granted 510(k) clearance for the Tono-Vera in May 2024; the device is also available in Europe and Canada. In a recent US study, a premarket version of the Tono-Vera showed a strong correlation and agreement in IOP measurements with the Goldmann-correlated intraocular pressure (IOPg) measurements from the ORA.10

Conclusion
The recent developments in alternative methods for IOP measurement have heralded an exciting time for both patients and glaucoma specialists who seek better ways of monitoring and understanding IOP behavior in glaucoma. However, studies are still necessary to further evaluate the accuracy of the measurements in heterogenic populations and in long-term glaucoma follow-up. For example, previous instruments, such as the early air-puff tonometers, have demonstrated that they are not reliable enough for clinical decision making,11 and portable tonometers frequently underestimate high IOPs and overestimate low IOPs.7 It is also important to note that IOP estimated with tonometers represents only a surrogate marker in glaucoma and not the “true” IOP (which can only be measured invasively). These estimations are then only clinically relevant if they are able to predict outcomes in the disease, such as visual field progression.12 Future studies should further investigate whether these novel methods are predictive of such outcomes in comparison to standard methods of tonometry.
References
1. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021;105(Suppl 1):1-169. doi:10.1136/bjophthalmol-2021-egsguidelines
2. Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95(5):627-629. doi:10.1136/bjo.2010.192922
3. Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012;130(12):1534-1539. doi:10.1001/archophthalmol.2012.2280
4. De Moraes CG, Mansouri K, Liebmann JM, Ritch R; Triggerfish Consortium. Association between 24-hour intraocular pressure monitored with contact lens sensor and visual field progression in older adults with glaucoma. JAMA Ophthalmol. 2018;136(7):779-785. doi:10.1001/jamaophthalmol.2018.1746
5. Koutsonas A, Walter P, Roessler G, Plange N. Implantation of a novel telemetric intraocular pressure sensor in patients with glaucoma (ARGOS study): 1-year results. Invest Ophthalmol Vis Sci. 2015;56(2):1063-1069. doi:10.1167/iovs.14-14925
6. Choritz L, Mansouri K, van den Bosch J, et al. Telemetric measurement of intraocular pressure via an implantable pressure sensor — 12-month results from the ARGOS-02 trial. Am J Ophthalmol. 2020;209:187-196. doi:10.1016/j.ajo.2019.09.011
7. Liu J, De Francesco T, Schlenker M, Ahmed II. iCare Home tonometer: a review of characteristics and clinical utility. Clin Ophthalmol. 2020;14:4031-4045. doi:10.2147/OPTH.S284844
8. Pronin S, Brown L, Megaw R, Tatham AJ. Measurement of intraocular pressure by patients with glaucoma. JAMA Ophthalmol. 2017;135(10):1030-1036. doi:10.1001/jamaophthalmol.2017.3151
9. Kratz A, Zbidat R, Kishner R, Cohen M, Shalata W, Goldberg I. Assessment of the iCare Home2, a new intraocular pressure self-measurement tonometer. J Glaucoma. 2023;32(11):926-929. doi:10.1097/IJG.0000000000002298
10. Youssif A, Jammal A, Malek D, Tseng H, Medeiros F. Comparison of intraocular pressure measurements between the new Tono-Vera portable tonometer and the Ocular Response Analyzer. Presented at: the American Glaucoma Society Annual Meeting; March 2-5, 2023; Austin, Texas.
11. Moseley MJ, Evans NM, Fielder AR. Comparison of a new non-contact tonometer with Goldmann applanation. Eye (Lond). 1989;3(Pt 3):332-337. doi:10.1038/eye.1989.48
12. Susanna BN, Ogata NG, Daga FB, Susanna CN, Diniz-Filho A, Medeiros FA. Association between rates of visual field progression and intraocular pressure measurements obtained by different tonometers. Ophthalmology. 2019;126(1):49-54. doi:10.1016/j.ophtha.2018.07.031









