An 88-year-old man was referred to me for treatment of severe primary open-angle glaucoma (POAG). The patient presented with IOPs of 19 mmHg OD and 20 mmHg OS; CCT of 505µm OD and 515µm OS; thin, hyperemic conjunctiva; PCIOL OU; and 0.9 CDR OU. His OCT RNFL revealed significant global thinning of 55µm OD and 49µm OS (Figure 1). He was being treated with latanoprost-netarsudil, brimonidine 0.1%, pilocarpine 2%, and dorzolamide-timolol OU.
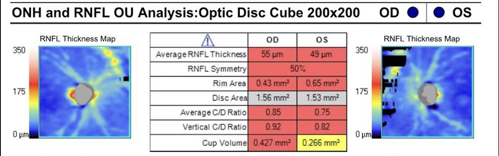
The patient reported that his vision had been diminishing over the past few years. His history was notable for CE/IOL/ECP OU in 2021, as well as SLT twice (most recently in March of 2022), which was ineffective. He was recently diagnosed with atrial fibrillation and was started on the anticoagulant apixaban.
Treatment Approach
As recently as 2 years ago, in a case like this, I would have proceeded to implant a non-valved tube shunt. However, there were several cautionary flags with this patient. While his advanced age and thin Tenon’s layer would place him at a higher risk for postoperative hypotony, I was more worried about my ability to manage a postoperative complication due to his self-admitted poor adherence with drops. I also noticed the patient rubbing his eyes often during our consultation, which could potentially break the conjunctival sutures or pull a tube ripcord. Finally, as the patient was fearful of driving, he could not promise consistent compliance with required post-op visits.
Classically, we glaucoma physicians will take one of two approaches to cases like this: We will either try to avoid surgery or jump right in. The wary specialist will fiddle with endless drop samples (since the medi-cations may not be affordable for the patient) and perhaps a goniotomy, and eventually realize they have become Sisyphus. We’ve all been there, especially in training. However, the aggressive specialists reading this may have their own regrets about performing any kind of conjunctival filtering surgery, given everything described in this patient’s case. Such a patient often gets stranded in no-man’s land and loses vision. We don’t have to let this happen now that we have the iStent infinite® (Glaukos).
I considered this patient an excellent candidate for iStent infinite, the first MIGS device FDA-cleared for standalone (and combo-cataract) surgery in patients with severe POAG who have failed prior medical and surgical interventions. Establishing a target IOP of 12 mmHg OU, I implanted iStent infinite OD with three stents, each approximately 2 clock hours apart. I also replaced the patient’s current medications with latanoprostene bunod and dorzolamide 2%, thereby reducing conjunctival hyperemia and achieving an IOP of 14 mmHg OU. Several months later, I performed the same procedure OS, reducing OS IOP to 16 mmHg OU.
Due to the patient’s difficulty adhering to a drop instillation regimen, I also injected sustained-release bimatoprost OU post-surgery. This achieved an IOP of 14 mmHg OU using dorzolamide 2% alone 1 year later. I expect to perform a micropulse CPC in the near future to reduce the IOP to 8-12 mmHg OU.
Multiple Benefits
As of this writing, I have performed 170 MIGS pro-cedures using the iStent infinite—of which 40 were performed in a standalone setting—and I can vouch for its multiple benefits. For one, it leaves your treatment options open. For example, if I’m aiming for a 3- to 6-point drop in IOP OU, I can expect good results using the iStent infinite alone.
Not only that, but this approach leaves the trabecular meshwork virtually untouched if my patient requires another angle-based surgery or even an iDose® TR (travoprost intracameral implant) 75 mcg in the future. And, of course, untouched conjunctiva remains for later insertion of a non-valved tube, if necessary.
A second advantage to the iStent infinite is its short learning curve. Although the device consists of three stents instead of two, I have found that the more I implant them during cataract surgery, the more confidence-inspiring it becomes to implant them as a standalone MIGS procedure. If you’ve performed iStent inject® W surgeries, your learning curve is likely complete.
Proven Safety and Efficacy
The iStent infinite® and its older siblings have an outstanding safety profile. Between the first-generation iStent® (2012), the iStent inject® (2018), the iStent inject® W (2020), and the iStent infinite (2022), we have more than 10 years of data to back up the device’s superior safety profile and long-term efficacy. Indeed, the IDE pivotal trial for the iStent infinite, which enrolled 72 subjects who had previously undergone an average of 2 unsuccessful incisional or cilioablative glaucoma surgeries and/or were unresponsive to an average of 3 topical IOP-lowering medications, found that 76.1% of all enrolled patients met the effectiveness endpoint of ≥20% reduction in median diurnal IOP (MDIOP) from baseline.1
In addition, for patients on the same or fewer medication(s) at baseline, the trial found that more than half achieved ≥30% MDIOP reduction without surgical interventions or other procedures. The study also found a favorable safety profile, with no explants, infection, or device-related interventions, or hypotony.1
Patient Counseling
When counseling patients on the need for stent-based MIGS, I tell them that we can now implant a tiny stent (“smaller than Lincoln’s nose on a penny”) to lower IOP instead of subjecting them to a trabeculectomy or non-valved tube shunt.
I also explain that iStents have been used to safely treat mild-to-moderate cases for over a decade, and we now have data showing severe cases like theirs can be treated the same way.
For me, the bottom line is that the iStent infinite offers a safe and effective standalone MIGS option for a broad range of patients, including severe refractory cases of POAG that previously could only be addressed by tubes or trabeculectomies.
Think of the thousands of pseudophakic patients forced to instill multiple drops several times a day. The iStent infinite can be your go-to MIGS because virtually any patient with severe POAG is an excellent candidate.
REFERENCE
1Sarkisian SR Jr, Grover DS, Gallardo MJ, et al. Effectiveness and Safety of iStent® infinite Trabecular Micro-Bypass for Uncontrolled Glaucoma. J Glaucoma. 2023;32(1):9-18.
THIS CONTENT IS SUPPORTED BY

IMPORTANT SAFETY INFORMATION:
iSTENT INFINITE® INDICATIONS FOR USE. The iStent infinite® Trabecular Micro-Bypass System Model iS3 is an implantable device intended to reduce the intraocular pressure (IOP) of the eye. It is indicated for use in adult patients with primary open-angle glaucoma in whom previous medical and surgical treatment has failed. CONTRAINDICATIONS. The iStent infinite is contraindicated in eyes with angle-closure glaucoma where the angle has not been surgically opened, acute traumatic, malignant, active uveitic, or active neovascular glaucoma, discernible congenital anomalies of the anterior chamber (AC) angle, retrobulbar tumor, thyroid eye disease, or Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure. WARNINGS. Gonioscopy should be performed prior to surgery to exclude congenital anomalies of the angle, PAS, rubeosis, or conditions that would prohibit adequate visualization that could lead to improper placement of the stent and pose a hazard. MRI INFORMATION. The iStent infinite is MRConditional, i.e., the device is safe for use in a specified MR environment under specified conditions; please see Directions for Use (DFU) label for details. PRECAUTIONS. The surgeon should monitor the patient postoperatively for proper maintenance of IOP. Three out of 61 participants (4.9%) in the pivotal clinical trial were phakic. Therefore, there is insufficient evidence to determine whether the clinical performance of the device may be different in those who are phakic versus in those who are pseudophakic. ADVERSE EVENTS. The most common postoperative adverse events reported in the iStent infinite pivotal trial included IOP increase ≥ 10 mmHg vs. baseline IOP (8.2%), loss of BSCVA ≥ 2 lines (11.5%), ocular surface disease (11.5%), perioperative inflammation (6.6%) and visual field loss ≥ 2.5 dB (6.6%). CAUTION: Federal law restricts this device to sale by, or on the order of, a physician. Please see DFU for a complete list of contraindications, warnings, precautions, and adverse events.
iDOSE ®TR INDICATIONS AND USAGE. iDose TR (travoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT). DOSAGE AND ADMINISTRATION. For ophthalmic intracameral administration. The intracameral administration should be carried out under standard aseptic conditions. CONTRAINDICATIONS. iDose TR is contraindicated in patients with active or suspected ocular or periocular infections, patients with corneal endothelial cell dystrophy (e.g., Fuch’s Dystrophy, corneal guttatae), patients with prior corneal transplantation, or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]), patients with hypersensitivity to travoprost or to any other components of the product. WARNINGS AND PRECAUTIONS. iDose TR should be used with caution in patients with narrow angles or other angle abnormalities. Monitor patients routinely to confirm the location of the iDose TR at the site of administration. Increased pigmentation of the iris can occur. Iris pigmentation is likely to be permanent. ADVERSE REACTIONS. In controlled studies, the most common ocular adverse reactions reported in 2% to 6% of patients were increases in intraocular pressure, iritis, dry eye, visual field defects, eye pain, ocular hyperaemia, and reduced visual acuity. PLEASE SEE FULL PRESCRIBING INFORMATION at www.glaukos.com/wp-content/uploads/2024/03/iDose-TR-Prescribing-Information.pdf. You are encouraged to report all side effects to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088. You may also call Glaukos at 1-888-404-1644.









