In recent years, glaucoma specialists have witnessed exciting therapeutic developments aimed at addressing important unmet clinical needs for patients. Topical agents often remain first-line therapies in the armamentarium, with demonstrated efficacy and safety results in the clinical trial setting. However, as many as 90% of patients struggle with adherence to their prescribed topical treatment regimen, resulting in suboptimal outcomes for up to 50%.1,2 In addition to nonadherence, other recognized limitations of topical treatment include fluctuations in intraocular pressure (IOP) control, challenges with dosing schedules and administration, and side effects such as ocular surface disease (OSD) and hyperemia. These often culminate in a negative impact to patients’ overall quality of life.3,4
Advancements in diagnostic technologies have facilitated earlier and more precise diagnosis, allowing glaucoma specialists to intervene earlier in glaucoma patients’ journeys and proactively consider more personalized approaches. In particular, managing glaucoma with microinvasive glaucoma surgery and sustained-release procedural pharmaceuticals has the potential to be transformational, reducing the burden of drop instillation for patients.
In December 2023, the FDA approved the iDose TR implant, a first-of-its-kind biocompatible titanium intracameral sustained-release implant that is designed to deliver a novel proprietary formulation of prostaglandin analog (travoprost) to the anterior chamber. The device's permanent J code will become effective July 1, 2024.5 The iDose TR delivery system includes the travoprost-releasing implant preloaded in a sterile, single-use inserter. In its entirety, the iDose TR implant measures 1.8 mm x 0.5 mm and includes 4 main parts: a drug reservoir, an elution membrane for controlling the rate of elution, the scleral anchor, and the titanium cap which holds the membrane in place (Figure 1). The implant is administered by internal approach through a small, clear corneal incision and is anchored through the trabecular meshwork into the sclera at the iridocorneal angle. Once affixed, iDose TR releases therapeutic levels of travoprost directly to target tissues, circumventing any issues associated with corneal permeability.

Based on the safety and efficacy of the phase 2b trial,6 2 pivotal, prospective, randomized, multicenter, double-masked, phase 3 trials were conducted.7 The studies randomly assigned 1,150 patients with open-angle glaucoma or ocular hypertension from 89 clinical sites to undergo 1 of 3 treatments: fast-eluting implant, slow-eluting implant, or sham surgery followed by twice-daily topical timolol ophthalmic solution 0.5%. Both phase 3 trials successfully achieved the prespecified primary efficacy endpoints through 3 months and demonstrated a favorable tolerability and safety profile throughout study follow-up. In the slow-elution implant arm, IOP reductions from baseline to 3 months were 6.6 mmHg to 8.4 mmHg, compared to 6.5 mmHg to 7.7 mmHg in the timolol control arm. Notably, at 12 months from baseline, 81% of patients in the slow-eluting group were completely free of IOP-lowering topical medications across both trials. The implant demonstrated excellent tolerability in the phase 2b and both phase 3 trials, with the most common ocular adverse reactions (2% to 6%) being IOP increase, iritis, dry eye, and visual field defects, most of which were mild and transient in nature. Additionally, 3-year data from the phase 2b study showed comparable changes from baseline in corneal endothelial cell counts in the implant and timolol control groups. The slow-eluting implant is currently marketed as iDose TR.
In addition to the phase 2b trial and the 2 phase 3 trials, a separate open-label, single-center trial was conducted to determine in-patient drug elution rate and aqueous humor exposure to travoprost free acid (TFA).8 At all time points through 24 months postoperative, mean TFA levels were above the maximum concentration level for topical travoprost; they were also above the minimum TFA level required for implant efficacy. At 24 months, the slow-eluting implants still had 16% of travoprost remaining, potentially indicating potential for drug delivery beyond 2 years.
iDose Pearls
Initial real-world experience can complement clinical trial data. Below, I will share my experience implanting iDose TR in patients with open-angle glaucoma and ocular hypertension patients. I will also share factors such as patient selection and clinical workflow and will provide insights about the nuances of this microinvasive injectable treatment.
Patient Selection
iDoseTR has a broad label, being FDA-approved for use in any stage of open-angle glaucoma and ocular hypertension. It can be implanted in patients with any glaucoma severity, any level of preoperative medication burden, any surgical history (or lack thereof), and in both standalone and combined-cataract procedures. My ideal patient is someone who needs IOP reduction but is noncompliant with or unable to use topical medications. It is a good sign if the patient has had good IOP-lowering response to a prostaglandin analog medication in the past, although this is not necessary. For my initial patients, I also prefer standalone cases (without cataract surgery).
Surgical Experience, Considerations, and Clinical Workflow
When integrating any new technology or procedure, adequate preparation of the surgeon, clinical personnel, and operating room team members is key to optimizing success and safety. To that end, when performing a procedure for the first time, I request a little extra time be added to the schedule. We also take time postprocedure to review the experience and discuss any specific challenges or ways to optimize future procedures. Incremental growth in all learning is important, so for the sake of patients, it’s critical we allocate time and resources to that activity.
Patient Positioning and Visualization
As with any angle-based procedure, proper patient positioning and gonioscopic angle visualization are imperative for iDose TR implantation. At the patient’s preoperative visit, I do a full gonioscopic examination at the slit lamp; this is to ensure open angle configuration, and sometimes leads me to adjust my incision and implantation locations. Immediately prior to the procedure, I do intraoperative gonioscopy in the operating room, and I position myself directly across from the implantation location. We must be disciplined about identifying and achieving the best view of the anterior chamber anatomy, including head adjustment and/or surgeon positioning as needed.
I’ve found that adequate magnification is incredibly important. I agree with the trainers’ advice that “90% of a successful angle procedure is getting the right view.” Even if these steps add extra minutes to the procedure, it is time well spent because it allows the implant to be placed smoothly in the correct location. Do not be in a hurry to implant before getting a proper view of the angle, as this leads to higher likelihood of misplacement and having to regrasp, reload, and retry again. In addition, the surgeon may want to consider the use of a topical miotic prior to the patient’s entry into the surgical suite to protect the natural lens while traversing the anterior chamber with the inserter during the procedure. Use of a miotic also can be considered during the initial learning period, while the surgeon is getting comfortable performing the procedure.
Procedural Pearls
At the start of the procedure, I fill the anterior chamber with cohesive viscoelastic to make the eye firm, which greatly facilitates implant placement. For the implantation itself, my advice is, slow down. First, ensure ideal visualization, as discussed above, then proceed through 4 distinct steps: (1) compress the middle of the trabecular meshwork (“dimple”); (2) return to neutral position; (3) open the graspers of the injector, and nudge the implant to either side to ensure it is firmly placed in the sclera; and, (4) slowly pull straight back from the implant.
Although the implant is designed to be effective in any angle quadrant, I typically place it superonasally, which cosmetically helps conceal the implant below the upper eyelid (Figure 2). After implantation, diligently remove all viscoelastic. While doing this, monitor to ensure that the implant does not wiggle or move excessively, because this could indicate inadequate anchoring into the scleral wall.
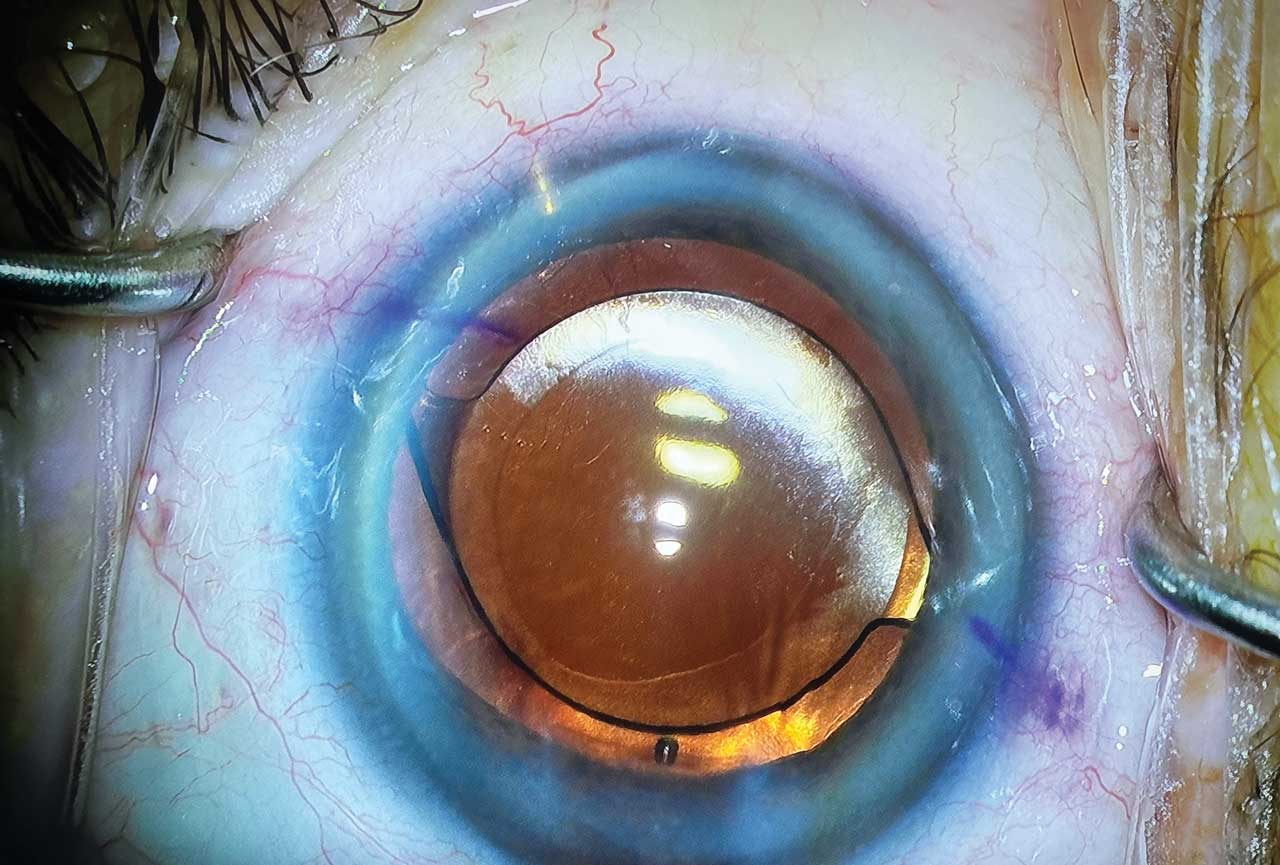
Postoperative Care
At the end of the case, I provide the patient with intracameral moxifloxacin for antibiotic coverage to help minimize the amount of postprocedural drops. I ask the patient to use a low-dose topical steroid 4 times a day for 4 days, and to keep that bottle for the second eye. Similar to my other ocular surgeries, I ask that patients refrain from heavy lifting or strenuous activity for 1 week; I do not apply a patch. Unless an issue arises, I typically see patients the next day to assess proper placement of the implant and measure their IOP. This information helps me decide how to best proceed with the rest of their glaucoma drop regimen, if they are still on other drops. Regarding patients’ glaucoma medications, I instruct patients to stop using their topical prostaglandin analog drops and possibly their adjunctive medications, depending on their prior IOP and glaucoma status, on the day prior to the procedure. I do not resume their medications after the procedure because I want time to assess where the IOPs will go. The implant allows me to “reset” care so we can add back therapy only as needed, to minimize the number of drops overall at the end.
Following the procedure, I prescribe a topical fluoroquinolone antibiotic 4 times a day for 1 week, and a topical nonsteroidal anti-inflammatory medication on a 4-week taper. Of note, I do not prescribe topical steroid eyedrops, as in my experience these are unnecessary because the procedure and implant are very well tolerated. Unless an issue arises, I typically see patients at 1 day, 1 week, and 1 month postoperative.
Final Thoughts
Procedural pharmaceuticals are a promising new addition to the glaucoma treatment armamentarium, designed to remove or lessen the burden of medication compliance from the patient while effectively lowering IOP. The recently approved iDose TR implant comes with a solid foundation of randomized, controlled, prospective, multicenter data showing strong efficacy and safety of the device in the clinical trial setting. In my real-world experience, I have found iDose TR to be effective and well tolerated. The implantation procedure is easily incorporated into my surgical day, and postoperative care is manageable. I look forward to continuing to offer this novel technology for the better care of my patients. GP
References
1. Zaharia AC, Dumitrescu OM, Radu M, Rogoz RE. Adherence to therapy in glaucoma treatment — a review. J Pers Med. 2022 Mar 22;12(4):514. doi:10.3390/jpm12040514
2. Nordstrom BL, Friedman DS, Mozaffari E, Quigley H, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4): 598-606. doi:10.1016/j.ajo.2005.04.051
3. Radcliffe NM, Shah M, Samuelson TW. Challenging the “topical medications-first” approach to glaucoma: a treatment paradigm in evolution. Ophthalmol Ther. 2023;12(6):2823-2839. doi:10.1007/s40123-023-00831-94.
4. Gelb L, Friedman DS, Quigley HA, et al. Physician beliefs and behaviors related to glaucoma treatment adherence: the Glaucoma Adherence and Persistency Study. J Glaucoma. 2008;17(8):690-698. doi:10.1097/IJG.0b013e31816b3001
5. Gallagher J. Permanent J-code assigned for iDose TR. Glaucoma Physician. April 19, 2024. Accessed April 22, 2024. https://www.glaucomaphysician.net/issues/2024/march/permanent-j-code-assigned-for-idose-tr/
6. Berdahl JP, Sarkisian SR Jr, Ang RE, et al. Travoprost Intraocular Implant Study Group. Efficacy and safety of the travoprost intraocular implant in reducing topical IOP-lowering medication burden in patients with open-angle glaucoma or ocular hypertension. Drugs. 2024;84(1):83-97. doi:10.1007/s40265-023-01973-7
6. Sarkisian SR, Ang RE, Lee AM, et al. Travoprost intracameral implant for open-angle glaucoma or ocular hypertension: 12-month results of a randomized, double-masked trial. Ophthalmol Ther. 2024;13(4):995-1014. doi:10.1007/s40123-024-00898-y
7. Glaukos data on file.