Glaucoma is characterized by optic nerve damage due to elevated intraocular pressure (IOP) and poses a risk of irreversible vision loss. Lowering IOP, medically or surgically, remains the main objective in glaucoma treatment. Produced by the ciliary body, the aqueous humour flows from the anterior chamber through 2 different routes:
The trabecular pathway, responsible for 70% of aqueous drainage. It involves the trabecular meshwork, Schlemm’s canal, and aqueous collector channels. In glaucoma, along with age-related changes in trabecular cells and the extracellular matrix, this outflow can decrease.1
In the uveoscleral route, the aqueous flows through the uveal trabeculum and the ciliary muscle bundles, eventually being drained through the choroid. This pathway initially accounts for 30% of aqueous drainage, but this can decrease with age, dropping to as low as 3%.2
Following pharmacologic and laser treatments, trabeculectomy remains the surgical standard for lowering intraocular pressure (IOP). However, secondary criteria of a meta-analysis published in 2013 in JAMA Ophthalmology evaluating the efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures show that this effective but irreversible intervention is associated with a higher incidence of short and long-term complications, such as hypotony, choroidal effusion, cataract, and shallow or flat anterior chamber.3
The development of minimally invasive glaucoma surgery (MIGS) procedures have changed the approach to surgical treatment strategies. For example, placement of metallic trabecular stents in Schlemm’s canal, such as the iStent devices (Glaukos) and Hydrus Microstent (Alcon) create a shortcut to outflow channels, bypassing the trabecular obstacle.4 Moreover, these surgeries are often performed alongside cataract extraction, further enhancing their pressure-lowering effect.
Cyclodialysis, first described by Holt in 1905, is a technique designed to create a direct connection between the anterior chamber (AC) and the uveoscleral supraciliary route. To prevent secondary closure of the cyclodialysis cleft, various devices have been placed either ab interno or ab externo.5
In the context of inflammation and endothelial cell loss (ECL), the role of supraciliary stents, particularly the CyPass Micro-Stent (Alcon), raises concerns. The device was withdrawn in 2018 due to issues regarding patient safety, rooted in 5-year data indicating elevated rates of ECL in some patients. This was reported to be related to the device not being inserted deep enough into the iridocorneal angle. This clearly illustrates that despite manufacturer recommendations and surgeon training, surgery remains a manual art and variations in surgical technique remain inevitable.
Avoiding Endothelial Cell Loss
Avoiding penetration of the AC and/or insertion of instruments and devices in the angle may decrease the postoperative inflammation level and eradicate the risk of ECL by design. The rationale of the cilioscleral interposition (CI) approach, and a corresponding Cilioscleral Interpositioning Device (CID) (Ciliatech), is based on the hypothesis that a localized permanent separation of the ciliary body from the sclera, close to the uveal trabeculum but without opening the AC, could significantly increase the uveoscleral outflow (USO) and lower IOP without the need to create artificial outflow channels.
This approach was developed after years of clinical observations following various types of glaucoma surgery, and was also supported by an in vivo animal experiment in 1989.6 This research aimed to measure the hydrostatic pressure of the suprachoroidal space in 18 eyes of cynomolgus monkeys, as compared to the AC and to the supraciliary area. The authors demonstrated that the main barrier to USO is not at the iris root but at the supraciliary level due to the contact between the sclera and the ciliary body.
These data raise the question of whether a localized suppression of this contact, facilitating outflow access into the suprachoroidal area, could increase USO and lower IOP. We sought to address this question by inserting a CID between the sclera and the ciliary body, close to the uveal trabeculum, without penetrating the AC.
Development of a Cilioscleral Interposition Device
A first-generation CID was designed and developed by Ciliatech and tested in 2 clinical trials named SAFARI (“SuprAciliary Filtration Alone Reduces IOP”). The SAFARI I and SAFARI II trials enrolled 42 patients with open-angle glaucoma, and the device was implanted as a standalone procedure.7-9
At the 24-month follow-up, the interim combined results of SAFARI I and II showed that mean IOP dropped by 38% and 81% of patients achieved IOP ≤18 mmHg. Eighty-five percent of patients were free from glaucoma medications. The reported side effects in the trials were few, transient, and generally mild.7-9
The second-generation CID (Figure 1) is a one-piece implant made of 26% hydrophilic acrylic, a material well established for its biocompatibility in ophthalmic surgery. It is designed with dimensions of 6 mm in length and 3.4 mm in width, its thickness ranging from 150 μm (anterior edge) to 600 μm (posterior edge).

This second-generation CID has been tested in a subsequent study. The SAFARI III clinical trial involved 57 patients with an equal proportion of primary open-angle glaucoma and primary angle-closure glaucoma, all performed as standalone procedures.
At the 12-month follow-up interim analysis, the outcomes demonstrated in the SAFARI III trial seem improved vs the previous generation. Mean IOP dropped by 41%, lowering the IOP from 23.5 mmHg to 13.9 mmHg, 90% of patients achieved IOP equal to or below 18 mmHg, and 88% of patients were free from glaucoma medications. Consistent with the results from SAFARI I and II, SAFARI III demonstrated a strong safety profile, reporting no major complications.10
Perhaps of greatest interest is that the efficacy and safety outcomes were comparable between both open-angle and narrow-angle patients, remembering that cataract extraction was not a confounding feature of this trial.
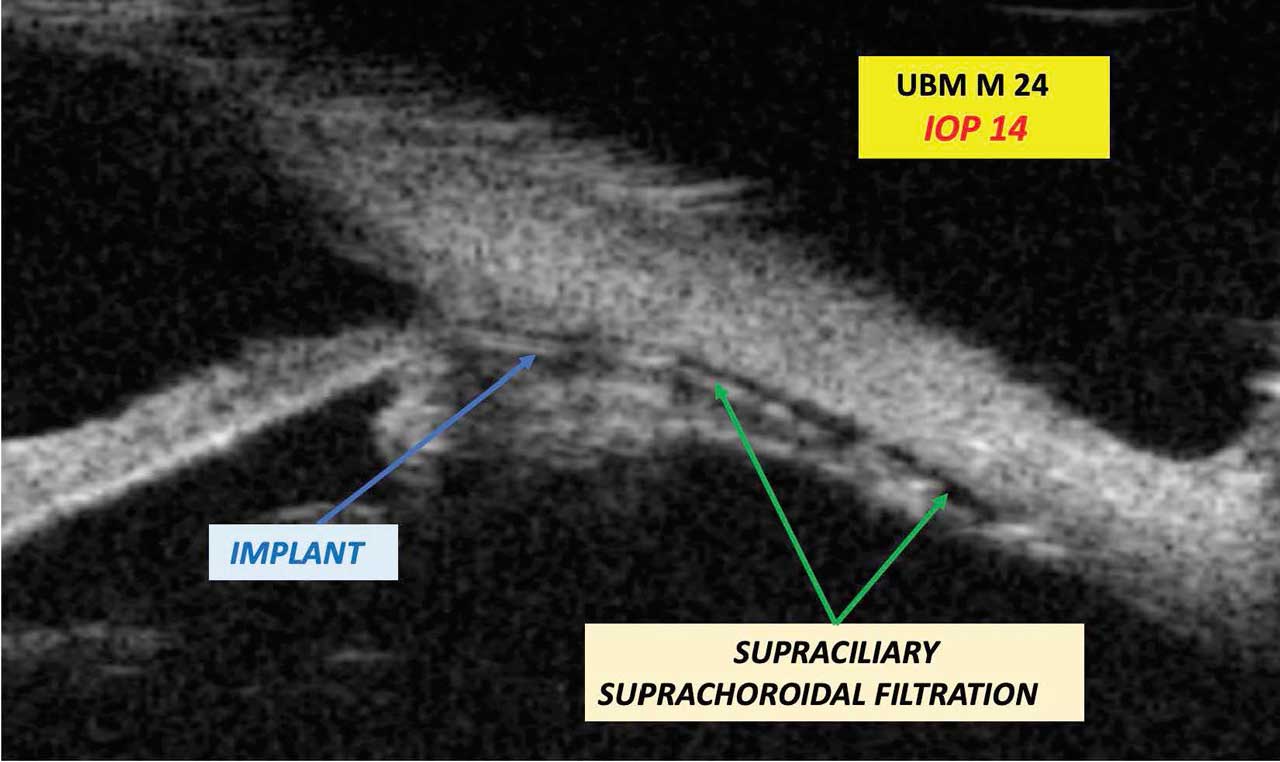
Ultrasound biomicrosopy (UBM) examinations were carried out as early as day 1, and then on day 7, month 1, month 3, month 6, month 12, and month 24 (Figure 2). No movement of the implant, no foreign body reaction, and no encapsulation were noted. Supraciliary and suprachoroidal filtration were demonstrably visible in approximately 60% of cases.
Surgical Technique
The surgical technique is atraumatic and physiologic outflow channels remain intact and uninterrupted. Two radial conjonctivotenonial and full-thickness scleral incisions allow the separation of the sclera from the ciliary body with the aid of a small quantity of cohesive ophthalmic viscosurgical device. The CID is then slid into position with forceps, and finally the incisions are tightly closed to prevent subconjunctival leakage. Involvement of the conjunctiva is limited to the incisions, and no additional adhesions are created.
Discussion and Conclusion
Progress in glaucoma treatment in the last 20 years is irrefutable. The increasing prevalence of the MIGS category has raised the bar toward more tailored disease management and lower side effects for patients. The risk-benefit ratio of surgery vs drugs or noninvasive techniques such as lasers has improved to the point that incisional surgery is no longer seen as the last solution when other treatment options have failed. However, despite scientific advances and industry innovation, there still remains room for additional research, improvements, and overall progress.
From a research perspective, the CID concept is supported by the publication by Emi et al6 and by years of clinical observations in patients. Beyond theory, the 3 SAFARI trials using the CI technique in more than 100 patients, and with up to 24-month follow-up, seem to support that USO enhancement without opening the AC is indeed a viable and effective surgical strategy. This stimulates additional interesting questions: what mechanisms drive the aqueous exit from the AC through the uveal trabeculum? Is the USO drainage capacity limited to 3% to 30% as commonly reported, or can it drain more? What mechanisms support the reduction of USO capacity with age? Can first signs of IOP increase result from USO decrease? In addition to the UBM examinations described above, further research likely involving OCT angiography to fully understand all the mechanisms behind IOP reduction through intrascleral and episcleral circulation will be interesting.
From the patient benefits perspective, the most prominent factor supporting the CI approach is the preservation of the AC and the conjunctiva. Preserving the AC eradicates the risk for ECL (both intraoperatively and postoperatively) and reduces post-operative inflammation and adverse events. This also means that the CID can be used for any angle conformation (eg, regardless of Shaffer grade) and finally opens up access to minimally invasive techniques for primary angle-closure glaucoma patients, while preserving their lens. In addition, preserving the conjunctiva means that conjunctival vessels can still drain aqueous, and most importantly it means that more invasive filtering surgeries can still be performed as needs arise in patients whose disease may continue to progress.
Cilioscleral interposition does not create any artificial egress for the aqueous (no hole through functional tissues, no bleb, no conduit, no aqueous derivation); therefore, it only relies on the eye’s natural anatomic and physiologic capacities to self-regulate. As such, its performance may be limited when the eye structure is damaged, which suggests that its optimal use should be early in the disease progression, in the mild to moderate stages, before filtering surgery becomes the only option. Another key advantage is that IOP is unlikely to go below physiologic levels and therefore the risk of hypotony is very low.
To the best of our knowledge, this is the first demonstration of the possibility to lower IOP in glaucoma without artificial egress, without entering the AC, and without creating a bleb. This approach based on physiological support seems to be an additional chance for our glaucomatous patients. While these results are very encouraging, more evidence is needed to confirm the clinical profile and utility of the CI approach. GP
References
1. Buffault J, Labbé A, Hamard P, et al. The trabecular meshwork: structure, function and clinical implications. A review of the literature. Article in French. J Fr Ophtalmol. 2020;43(8):779-793.
2. Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52-59. doi:10.2174/1874364101004010052
3. Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573-1582. doi:10.1001/jamaophthalmol.2013.5059
4. Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811-821. doi:10.1016/j.ophtha.2019.03.006
5. Denis P, Hirneiß C, Durr GM, et al. Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial. Br J Ophthalmol. 2022;106(1):65-70. doi:10.1136/bjophthalmol-2020-316888
6. Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30(2):233-238.
7. Voskanyan L. Supraciliary drainage without entering the anterior chamber and without bleb: a totally new glaucoma surgery concept. Poster presented at: the European Society of Cataract and Refractive Surgeons annual meeting; September 18, 2022; Milan, Italy; poster PP15.10.
8. Voskanyan L, Papoyan V, Sourdille P, Benoit O, Miroyan H. Supraciliary drainage without entering the anterior chamber and without bleb: 24 months follow-up results. Poster presented at: World Glaucoma Congress; June 2023; Rome, Italy; poster 842-P. Accessed January 12, 2024. https://www.postersessiononline.eu/173580348_eu/congresos/WGC2023/aula/-P_842_WGC2023.pdf
9. Voskanyan L, et al. 2-years outcomes of a novel cilioscleral interposition device: enhancing outflow without entering the anterior chamber. Poster presented at the European Society of Cataract and Refractive Surgeons annual meeting; September 2023; Vienna, Austria; Eposter PO0858.
10. Ciliatech presents preliminary one-year study results on treating open and narrow angle glaucoma with CID at Ophthalmology Futures Symposium. News release. September 7, 2023. Accessed January 12, 2024. https://ala.associates/clinical/ciliatech-presents-preliminary-one-year-study-results-on-treating-open-and-narrow-angle-glaucoma-with-cid-at-ophthalmology-futures-symposium/









