Nova Eye Medical’s iTrack 250A broke new ground when it introduced canaloplasty into the treatment paradigm for primary open-angle glaucoma in 2008.
“The device works incredibly well, but it requires using microforceps to maneuver the microcatheter within the Schlemm’s canal,” says Jordan Green, chief technology officer at Nova Eye Medical in Fremont, California. “We decided to redesign the device to eliminate the need for microforceps and to achieve better control and ergonomics.”
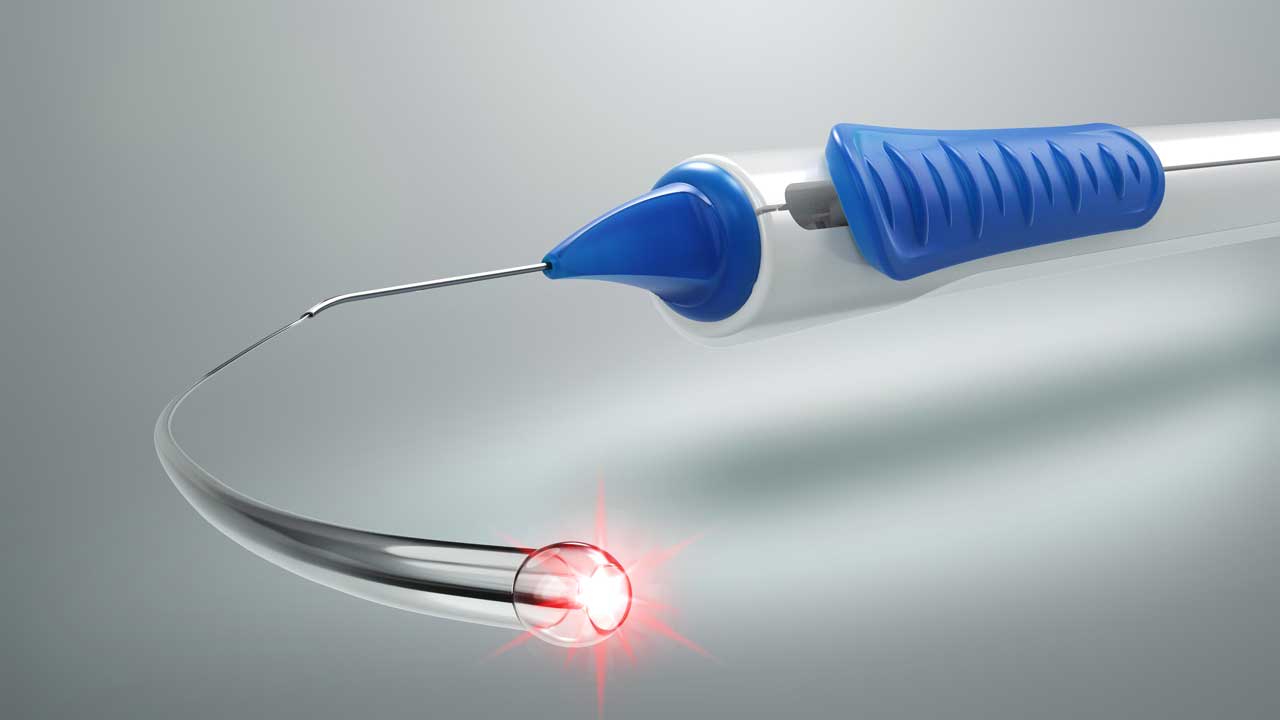
The new device, called the iTrack Advance, features a novel delivery platform that doesn’t require manipulating the microcatheter with forceps. Like its predecessor, the iTrack Advance microcatheter is an intricately engineered device that allows for microcatheterization and pressurized viscodilation (delivering +100 μl of ophthalmic viscosurgical device) to be performed across the entire 360° of the canal. The iTrack Advance also has an illuminated microcatheter tip, enabling users to visualize its location in the canal at all times.
Arsham Sheybani, MD, associate professor of ophthalmology and visual sciences at Washington University in St. Louis, Missouri, an early adopter and medical advisory board member for Nova Eye Medical, says, “The illuminated tip always lets you know where you are in the eye, which prevents many undesired complications. Without it, there are multiple risks, such as ending up in the posterior segment or having it come out of the canal.”
Designed to deliver hyaluronic acid-based viscoelastic, the iTrack Advance’s infusion mechanism is unique in that it is decoupled from the drive mechanism of the handpiece. “This allows surgeons to choose how much pressurized viscoelastic to deliver and when,” Green explains. For example, if a surgeon hits a blockage in the canal during intubation they can deliver a few microboluses of the viscoelastic to help dilate the canal and pass the obstruction.
The pressurized injection of viscoelastic also further stretches the trabecular meshwork while simultaneously dilating Schlemm’s canal and the collector channels. Surgeons may choose to monitor the blanching effect that is typically observed in the scleral plexus — signaling improved outflow facility — and increase the rate of delivery of viscoelastic if they don’t observe sufficient blanching.
Sparing Tissue
Canaloplasty does not remove tissue or require a stent to be inserted. With the iTrack Advance, surgeons can open the canal, flush out the conventional outflow pathway, and see reductions in IOP and medication use while keeping their treatment options open for the future and keeping the natural physiological pathways intact, Green says. It doesn’t preclude subsequent surgeries.
“More invasive procedures, such as filtration surgeries, remain an option because canaloplasty doesn’t alter anything but only improves the natural outflow,” says Analisa Arosemena, MD, glaucoma specialist at Aran Eye Associates in Miami, Florida, who was an early adopter of the device.
Ergonomics
The iTrack Advance was designed with ergonomics in mind. The curvature of the handpiece falls naturally into the hand, from the shape and texture of the slider, to the shape of the nozzle of the rotatable cannula that allows users to feel the device’s rotation angle.
“Typically, we use one hand to hold the gonioprism for visualizing the angle, while the other hand carries out the procedure,” explains Dr. Arosemena. “However, with the new iTrack Advance handpiece, advancing the microcatheter is made easier by simply sliding the actuator, giving greater control.”
The device’s design makes canaloplasty easier to learn, as well. “This will likely encourage more surgeons, including those who specialize in cataract and comprehensive surgeries, to try it,” Dr. Arosemena says.
Clinical Applications
The iTrack Advance can be used in early-stage patients on 1 or 2 medications. Surgeons can perform canloplasty before or after a phacoemulsification to clean out the canal, the collector channels, and trabecular meshwork to improve outflow facility and aim to re-establish the natural flow of aqueous humor through the conventional outflow pathway.
The iTrack Advance is cleared as a standalone procedure. Surgeons may use it for mild to moderate and even later-stage glaucoma. Ultimately, the device enables surgeons to work with patients’ physiology, not against it, in reducing IOP, Green concludes. GP








