There are many challenges in medical management of glaucoma, the majority of which center around patient adherence, ability, and reliability. Adherence is a critical link between effective treatment and successful management; however, adherence to glaucoma medications varies, with estimates of medication non-adherence among glaucoma patients ranging from 30% to 80%.1 Reliability is also a critical variable. Most glaucoma patients are elderly, which can be associated with reduced manual dexterity from musculoskeletal pathology, tremor and memory impairment due to neurologic disease, and low vision due to ocular comorbidities — all of which reduce treatment reliability. Sustained-delivery treatments offer a means of decreasing the impact of these patient-dependent factors. This article will offer a review of sustained-delivery glaucoma treatments, ranging from drug-eluting contact lenses to surgically implanted drug-delivery systems.
Ocular Adnexa
One approach to sustained-delivery glaucoma treatment targets the ocular adnexa, including the conjunctival fornix, the nasolacrimal system, and the subconjunctival space. The first drug-delivery system to utilize the adnexa was the Ocusert (Alza Corporation), a pilocarpine-containing, polymembrane unit, approved in the 1970s. Though effective, this device was eventually taken off the market due to difficulty of insertion, foreign-body sensation, and low retention.3
Conjunctival Fornix Inserts
The Bimatoprost Ocular Ring (Bim Ring; Allergan) is a preservative-free silicone-polymer matrix insert, which, like the Ocusert, occupies the conjunctival fornix. The BIM Ring was designed to release bimatoprost over a 6-month period. Drug elution starts at 35 μg/day, decreasing to 6 μg/day by 6 months (for comparison, topical bimatoprost 0.03% is approved for once-daily use with a concentration of about 9 μg/day). In phase 2 trials, patients showed a clinically relevant and sustained IOP reduction over a 6-month period; however, criteria for noninferiority were not met when compared to topical timolol.3 The primary retention rate at 6 months was 88.5%, and the adverse effect profile was similar to that of topical drop delivery.4 Phase 3 trials have not been conducted.
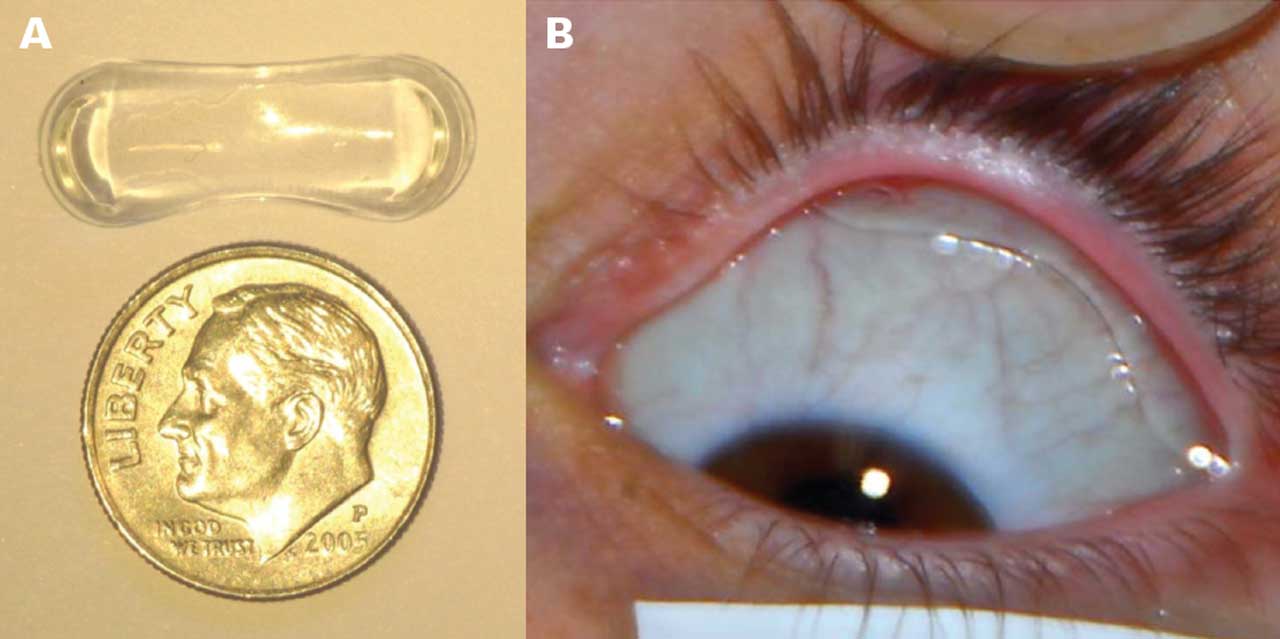
The Topical Ophthalmic Drug Delivery Device (TODDD; Amorphex Therapeutics) (Figure 1) also lies within the conjunctival fornix. This device is a soft polymer drug depot that floats in the superior fornix beneath the upper eyelid and provides sustained drug delivery for more than 90 days. Animal studies showed a 37% IOP reduction from baseline when loaded with timolol5 or latanoprost,6 and in a human safety and tolerability study, the retention rate was found to be 70% with no safety concerns after 4 weeks of wear.7 Additional human studies on timolol, prostaglandin analogs, and combination timolol and prostaglandin analogue, are ongoing.
Punctal Plugs
The nasolacrimal system offers another noninvasive option for sustained medication delivery. The Evolute platform (Mati Therapeutics) uses an L-shaped silicone punctal plug with a latanoprost core (Figure 2). In phase 2 trials, it demonstrated a statistically significant mean IOP reduction from baseline by about 22% at 1 month,8 and was found to have a similar rate of adverse events as that reported with commercial punctal plugs, with a retention rate in the inferior punctum of 92% to 96%.9 Ocular Therapeutix designed its OTX-TP device, a punctal-based platform with a polyethelene glycol resorbable hydrogel rod impregnated with fluorescein (to aid in visualization) and embedded with travoprost to deliver medication over 3 months. In a phase 3 trial, OTX-TP did not demonstrate superiority to a nondrug-eluting punctal plug, and had a 3-month retention rate of 48%.10 The OTX-TP system is no longer being developed.11

Contact Lenses
The tear film can also be utilized for sustained medication delivery by means of drug-eluting contact lenses. LLT-BMT1 (MediPrint Ophthalmics) is a drug-eluting contact lens that releases bimatoprost. In the SIGHT-1 phase 2a clinical study, no significant adverse effects were reported, and the lenses were found to have 100% tolerability, with a rate of hyperemia that was lower than that of users of bimatoprost drops.12 MediPrint Ophthalmics recently completed the SIGHT-2 phase 2b clinical study, which showed a clinically meaningful IOP reduction of 14% to 19% from baseline over 3 weeks of contact lens wear, which was comparable to topical timolol.13
Joseph Ciolino, MD, at Massachusetts Eye and Ear Infirmary has also developed a drug-eluting contact lens, which delivers latanoprost. In preliminary studies on glaucomatous monkeys, this contact lens was found to be noninferior to daily latanoprost.14 Safety and efficacy trials are currently under way.
Subconjunctival Injections
The subconjunctival space offers a somewhat minimally invasive space for depot medication delivery. Vi-Sci’s Eye-D latanoprost insert (BioLight Life Sciences) is a device in development which involves the in-office insertion of an implant that releases latanoprost into the subconjunctival space over 3 months. In a phase 1/2a trial, the Eye-D insert was found to lower IOP by 24% at 12 weeks.15
Benefits and Barriers
The benefits of using the ocular adnexa for sustained-delivery glaucoma treatment include a relative lack of invasiveness and the potential for in-clinic administration. Punctal plugs and contact lenses have already been used extensively in ophthalmic care, and therefore safety and acceptance may be less of a barrier to use. In addition, with devices such as punctal plugs and contact lenses, there is the capacity to adjust sizing and configuration to fit most patients’ anatomy. However, many of these devices exert their effect by constant exposure of the ocular surface to medication, which does not address the issue of side effects that are common with topical glaucoma medications. Adnexal implants can also become dislodged or fall out; if this is not obvious, it could leave patients unaware of a lack of IOP control. Further, these devices are foreign objects, which introduces the potential for irritation, inflammation, and infection.
Intraocular Options
Another approach to sustained-delivery treatment targets the intraocular space via the anterior or posterior chamber.
Iridocorneal Angle Injectables
Durysta (Allergan) is a rod-shaped, biodegradable polymer matrix containing 10 µg of bimatoprost, and it is currently the only FDA-approved sustained-delivery glaucoma therapy. Durysta can be inserted into the iridocorneal angle at the slit lamp or in the operating room and is designed to dissolve over 3 to 4 months, although its IOP-lowering effect can last months to years.16 In the 2 ARTEMIS phase 3 trials, Durysta lowered mean IOP by approximately 30% over the 12-week efficacy period.17 Due to corneal endothelial loss with multiple implants, Durysta is approved only for single application.
The OTX-TIC (Ocular Therapeutix) is a biodegradable hydrogel platform with travoprost-loaded microparticles, designed for insertion into the iridocorneal angle, degrading over 5 to 7 months. Phase I clinical trials found sustained IOP lowering of 6 months or longer, without any serious ocular adverse events. Phase 2 trials are currently ongoing.18
ENV515 travoprost XR created by Envisia Therapeutics is an injectable, biodegradable implant designed to release travoprost into the iridocorneal angle, using novel PRINT technology. In phase 2 trials, a single implant lowered IOP by a mean of 25% from baseline over 11 months, with no loss of efficacy over that period.19
Trabecular Meshwork Implants
The iDose TR (Glaukos) (Figure 3) is a sustained-delivery system that was FDA approved in December 2023 for the treatment of glaucoma. It is a titanium travoprost-eluting device, implanted in the trabecular meshwork and anchored to sclera. The iDose is designed to deliver long-duration drug therapy, though in clinical studies, the IOP-lowering effect was found to last out to 36 months in 68% of patients in the fast-release arm and 70% in the slow-release arm.20 Unlike Durysta, iDose TR requires surgical placement through a clear corneal incision in an outpatient setting. The iDose demonstrated a favorable safety profile.21

Intraocular Lens–based Devices
The SpyGlass system (SpyGlass Pharma) (Figure 4) is a novel device developed to be used in conjunction with the most widely used intraocular implant, the intraocular lens (IOL). The SpyGlass system attaches drug-eluting pads to the optic-haptic junction of an IOL. The IOL is then inserted through a standard 2.4-mm clear corneal incision into the capsular bag, where the drug-eluting pads remain outside the visual axis and continuously deliver medication directly into the aqueous for up to 3 years. The SpyGlass IOL with bimatoprost-eluting pads demonstrated a 45% mean reduction in IOP at 9 months, and phase 1/2 clinical trials have recently begun.22

Benefits and Barriers
Intraocular-based sustained-delivery systems for glaucoma management offer the benefit of avoiding medication side effects associated with topical exposure, as well as minimizing the risk of retention or inadvertent loss associated with implants in the adnexa. However, as these devices require entry into the intraocular space, they introduce a risk of endophthalmitis or corneal endothelial cell loss, as well as the potential for device migration and damage to surrounding intraocular structures. These devices also cannot be modified for an individual patient’s anatomy, and in some cases are only designed for a single use.
Conclusion
Just as no perfect glaucoma treatment exists, neither does the perfect sustained drug-delivery platform. However, with many exciting options under development, near approval, or already FDA-approved, we inch closer to being able to provide patients more treatment options with less reliance on patient adherence, ability, and reliability. GP
References
1. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53 Suppl1:S57-S68. doi:10.1016/j.survophthal.2008.08.002
2. Rupenthal ID. Drug-device combination approaches for delivery to the eye. Curr Opin Pharmacol. 2017;36:44-51. doi:10.1016/j.coph.2017.08.003
3. Brandt JD, Sall K, DuBiner H, et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology. 2016;123(8):1685-1694. doi:10.1016/j.ophtha.2016.04.026
4. Brandt JD, DuBiner HB, Benza R, et al. Long-term safety and efficacy of a sustained-release bimatoprost ocular ring. Ophthalmology. 2017;124(10):1565-1566. doi:10.1016/j.ophtha.2017.04.022
5. Leahy CD, Ellis EJ, Ellis JY, Crawford KS. Efficacy of a topical ocular drug delivery device (TODDD) for the treatment of glaucoma by telemetric measurement of IOP in the normal rabbit. Invest Ophthalmol Vis Sci. 2007;48:5861.
6. Crawford K, Ellis J, Rulander J, et al. Sustained delivery of prostaglandin from drug-containing depots using ocular rings in beagles. Invest Ophthalmol Vis Sci. 2013;54:5073.
7. Leahy CD, Gutner R, Varner W, et al. Continuous wear non-invasive device for sustained ocular drug delivery. Invest Ophthalmol Vis Sci. 2014;55:481.
8. Goldberg DF, Williams R. A phase 2 study evaluating safety and efficacy of the Latanoprost Punctal Plug Delivery System (L-PPDS) in subjects with ocular hypertension (OH) or open-angle glaucoma. Invest Ophthalmol Vis Sci. 2012;53:5095.
9. Singh RB, Ichhpujani P, Thakur S, Jindal S. Promising therapeutic drug delivery systems for glaucoma: a comprehensive review. Ther Adv Ophthalmol. 2020;12:2515841420905740. doi:10.1177/2515841420905740
10. Ocular Therapeutix announces tipline results of phase 3 clinical trial of OTX-TP for the treatment of glaucoma. News release. May 20, 2019. Accessed January 8, 2024. https://www.biospace.com/article/releases/ocular-therapeutix-announces-topline-results-of-phase-3-clinical-trial-of-otx-tp-for-the-treatment-of-glaucoma/
11. Kesav NP, Young CEC, Ertel MK, Seibold LK, Kahook MY. Sustained-release drug delivery systems for the treatment of glaucoma. Int J Ophthalmol. 2021;14(1):148-159. doi:10.18240/ijo.2021.01.21
12. MediPrint Ophthalmics announces successful completion of its SIGHT-1 phase 2a clinical study. News release. March 16, 2021. Accessed January 8, 2024. https://mediprintlens.com/mediprint-ophthalmics-announces-successful-completion-of-its-sight-1-phase-2a-clinical-study/
13. MediPrint Ophthalmics announces promising results from its SIGHT-2 phase 2b group 1 clinical study. June 8, 2023. Accessed January 8, 2024. https://mediprintlens.com/mediprint-ophthalmics-announces-promising-results-from-its-sight-2-phase-2b-group-1-clinical-study/
14. Ciolino JB, Ross AE, Tulsan R, et al. Latanoprost-eluting contact lenses in glaucomatous monkeys. Ophthalmology. 2016;123(10):2085-2092. doi:10.1016/j.ophtha.2016.06.038
15. BioLight Reports Successful Results in Phase 1/2a Clinical Trial for Glaucoma Insert. News release. July 24, 2017. Accessed January 8, 2024. https://www.prnewswire.com/news-releases/biolight-reports-successful-results-in-phase-12a-clinical-trial-for-glaucoma-insert-636305063.html
16. Medeiros FA, Sheybani A, Shah MM, et al. Single administration of intracameral bimatoprost implant 10 µg in patients with open-angle glaucoma or ocular hypertension. Ophthalmol Ther. 2022;11(4):1517-1537. doi:10.1007/s40123-022-00527-6
17. Allergan receives FDA approval for Durysta (bimatoprost implant) the first and only intracameral biodegradable sustained-release implant to lower intraocular pressure in open-angle glaucoma or ocular hypertension patients. News release. March 5, 2020. Accessed January 8, 2024. https://www.prnewswire.com/news-releases/allergan-receives-fda-approval-for-durysta-bimatoprost-implant-the-first-and-only-intracameral-biodegradable-sustained-release-implant-to-lower-intraocular-pressure-in-open-angle-glaucoma-or-ocular-hypertension-patients-301017349.html
18. Ocular Therapeutix presents data demonstrating a reduction in IOP in patients treated with OTX-TIC. News release. February 7, 2020. Accessed January 8, 2024. https://www.businesswire.com/news/home/20200207005064/en/Ocular-Therapeutix%E2%84%A2-Presents-Data-Demonstrating-a-Clinically-Meaningful-Reduction-in-Intraocular-Pressure-in-Patients-with-Primary-Open-Angle-Glaucoma-or-Ocular-Hypertension-Treated-with-OTX-TIC-at-Glaucoma-360-Conference
19. Masnberger SL, Conley J, Verhoever RS, et al. Interim analysis of low dose ENV515 tavoprost XR with 11 month duration followed by dose escalation and 28 day efficacy evaluation of high dose ENV515. Invest Ophthalmol Vis Sci. 2017;58:2110.
20. Glaukos’ iDose TR demonstrates sustained IOP reduction and favorable safety profile over 36 months in phase 2b study. News release. January 11, 2022. Accessed January 8, 2024. https://investors.glaukos.com/investors/news/news-details/2022/Glaukos-iDoseTR-Demonstrates-Sustained-IOP-Reduction-and-Favorable-Safety-Profile-Over-36-Months-in-Phase-2b-Study/default.aspx
21. Glaukos announces FDA approval of iDose TR (travoprost intracameral implant). News release. December 14, 2023. Accessed January 8, 2024. https://investors.glaukos.com/investors/news/news-details/2023/Glaukos-Announces-FDA-Approval-of-iDoseTR-travoprost-intracameral-implant/default.aspx
22. SpyGlass Pharma unveils 6-month data from the first-in-human trial of its innovative drug delivery platform for chronic eye conditions. News release. February 3, 2023. Accessed January 8, 2024. https://www.globenewswire.com/en/news-release/2023/02/03/2601509/0/en/SpyGlass-Pharma-Unveils-6-Month-Data-from-the-First-In-Human-Trial-of-Its-Innovative-Drug-Delivery-Platform-for-Chronic-Eye-Conditions.html










