Elevated intraocular pressure (IOP) is the main risk factor for both the development and the progression of glaucoma, and it remains the only factor that current therapies can effectively manage.1 Surgical options such as trabeculectomy and tube shunt implantation are well established but highly invasive therapies for lowering IOP when medical therapy and/or selective laser trabeculoplasty interventions are not sufficient.2 Although these glaucoma surgeries successfully lower IOP, they are also associated with high complication rates, including hypotony and bleb associated complications such as blebitis.2,3 Due to these concerns, an alternative treatment strategy that avoids the creation of filtering blebs has been sought.4
Because the uveoscleral pathway accounts for 5% to 30% of aqueous humor outflow, the supraciliary space has been targeted for innovative drainage strategies, including microinvasive glaucoma surgeries (MIGS).5 Supraciliary MIGS devices can access this space through an ab interno approach, which involves a clear corneal incision and intraoperative gonioscopy.6
Use of the supraciliary space to enhance outflow is not a new concept. More than a century ago, cyclodialysis clefts were used to increase aqueous humor drainage. However, outcomes were unpredictable, the risk of hypotony was high, and eventually the cleft would close. As trabeculectomy and other filtering surgeries were refined to offer consistent IOP control with fewer risks, cyclodialysis procedures fell out of favor.7
In 2017, the CyPass microstent (Alcon) became the first ab interno suprachoroidal MIGS device approved by FDA.8 The COMPASS study, as well as real-world results, showed CyPass was effective at lowering IOP. However, concerns soon arose regarding the loss of endothelial cells.9 The COMPASS XT study evaluated patients that had completed the 24-month COMPASS study, following them up to 60 months postoperatively, and reported a 20.4% endothelial cell loss with CyPass compared to 10.1% in controls at 60 months. Also, 3 cases of corneal edema were reported.10 Due to the concern for safety based on this study, the manufacturer decided to voluntarily withdraw the device in August 2018.
The iStent Supra (Glaukos) is a heparin-coated polyethersulfone stent designed to facilitate the flow of aqueous humor to the supraciliary space. It was approved for use in the European Union and some other countries outside the United States by 2017. A series of FDA investigational device exemption (IDE) trials were completed between 2017 and 2024. However, the iStent Supra program in the United States has stalled, making its future availability uncertain.11
Currently, there are 2 other approaches to access the supraciliary space. One is the MINIject (iStar Medical), an implant that is already approved for use in the European Union, United Kingdom, and Australia and is currently being investigated in the United States. The other is bioscaffolded cyclodialysis with scleral allograft, which uses Iantrek’s AlloFlo Uveo Bio-Spacers to create a cyclodialysis cleft. The spacers are delivered with Iantrek’s AlloSert Uveo (formerly CycloPen), a device that has received FDA approval.
MINIject
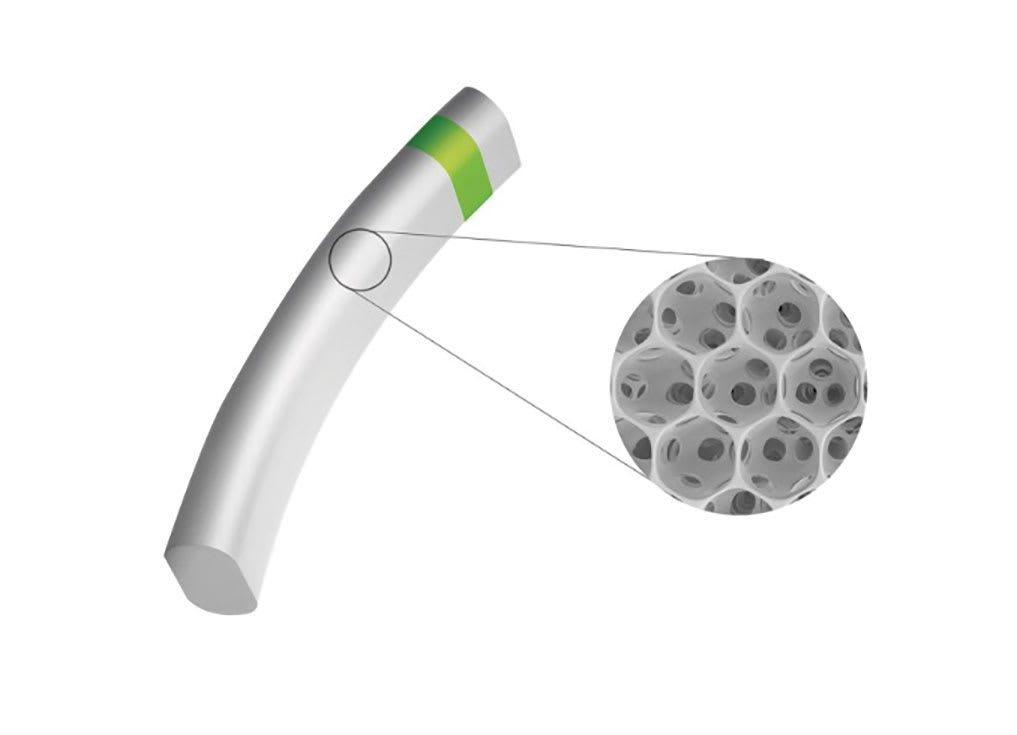
Figure 1. The MINIject device features a silicone honeycomb-like matrix that facilitates aqueous humor outflow. © iSTAR Medical.
The MINIject system includes a glaucoma drainage implant along with a delivery system designed to deliver the implant into the supraciliary space. The implant is 5 mm long, constructed from medical-grade silicone material, and is designed to conform to the anatomic features of the supraciliary space.12 The implant is made up of interconnected hollow spheres (Figure 1), resulting in 70% of the device’s total volume being empty.13
To place the MINIject, a 2.2 mm peripheral temporal corneal incision is created opposite the intended implantation site and the MINIject is advanced through the anterior chamber. A gonioprism is used for visualization, and the sheath tip is guided between the ciliary body and scleral spur until the green mark on the implant is flush with the ciliary spur, leaving the device protruding 0.5 mm into the anterior chamber.13
The device has been available in Europe since November 2021. In the United States, its safety and effectiveness is currently being evaluated in the STAR-V clinical trial.8 This is a prospective, multicenter, masked trial involving nearly 1,000 pseudophakic subjects age ≥46 years, diagnosed with primary open-angle glaucoma (POAG) who are candidates for medical therapy, laser treatment, or glaucoma filtering surgery. The primary endpoints are the proportion of subjects with ≥20% decrease from baseline in unmedicated diurnal IOP at 24 months and change from baseline in mean unmedicated diurnal IOP at 24 months.
In a separate trial that began enrolling patients in March 2025, the MINIject is being compared to the Hydrus Microstent (Alcon). MINHY is a prospective, randomized clinical study evaluating postoperative outcomes in patients with chronic glaucoma undergoing cataract surgery in combination with 1 of these MIGS devices. The study plans to include 152 patients who meet eligibility criteria, including the need for surgery to reach target intraocular pressure (IOP) and the presence of visually significant cataract. Participants are randomized to receive either a MINIject implant or a Hydrus Microstent. Both groups will undergo standard phacoemulsification cataract surgery. The primary outcome measure is mean IOP 1 year postoperatively, with follow-up extending to 5 years to assess safety, efficacy, imaging outcomes, and quality of life.14
The results of MINIject implantation in patients with medically uncontrolled POAG were evaluated in a study involving 37 patients—27 who underwent combined phacoemulsification with MINIject and 10 who received standalone MINIject. The mean preoperative intraocular pressure (IOP) was 17.95±4.75 mmHg on 2.16±1.12 glaucoma medications, which significantly decreased to 14.58±4.55 mmHg on 0.69±0.98 medications at 24 months. No significant difference in mean IOP was observed between the combined and standalone groups at 24 months.12
In another study involving 25 patients with uncontrolled glaucoma, the mean baseline IOP of 23.2±2.9 mmHg on 2.0±1.1 glaucoma medications decreased to 13.8±3.5 mmHg on 1.0±1.3 medications 24 months after MINIject implantation. Mean central endothelial cell density (ECD) decreased by 5% after implantation.15
Bioscaffolded Cyclodialysis
AlloFlo Uveo is the first homologous, bioconforming solution engineered specifically to enhance the uveoscleral outflow pathway through scleral reinforcement, offering a hardware-free, naturally derived alternative for glaucoma patients.16 The procedure, performed with the AlloSert Uveo system (Figure 2) uses a 5-mm x 500-μm microtrephined allogeneic scleral bioscaffold derived from minimally processed, acellular donor scleral tissue obtained from certified eye banks and screened for infectious disease.16

Figure 2. The AlloSert Uveo delivery system is used to implant AlloFlo Uveo Bio-Spacers, which are designed to maintain a cyclodialysis cleft for sustained uveoscleral outflow. Image courtesy Iantrek.
The technique begins with the ab interno creation of a cyclodialysis cleft that spans 1 to 2 clock hours, guided by gonioscopic visualization. Viscocycloplasty with cohesive viscoelastic is performed to expand the cleft, followed by the delivery of the bioscaffold using Iantrek’s AlloSert Uveo device. Proper placement is confirmed with gonioscopy, ensuring the proximal end of the scaffold is flush with the iris root. The viscoelastic is then evacuated.16
In one study, 31 eyes with POAG and visually significant cataracts underwent bioscaffolded cyclodialysis with phacoemulsification. At 12 months postoperatively, mean IOP decreased from 21.9±4.92 mmHg on 1.22±1.29 glaucoma medications to 12.62±2.63 mmHg on 0.55±0.52 medications.17 A separate case series of 117 eyes treated with this approach demonstrated a 27.1% mean IOP reduction at 12 months, with greater efficacy in eyes with baseline IOP >21 mmHg (n=45), which experienced a 39.7% decrease in IOP and a drop in glaucoma medications to 0.8±0.9. No unanticipated or serious adverse events were reported.18
In May 2025, Iantrek reported that more than 2,000 AlloFlo Uveo procedures had been performed in the United States, a milestone reached ahead of the product’s full commercial launch, which is planned to coincide with the 2025 meeting of the American Academy of Ophthalmology (AAO).18
Conclusion
The supraciliary space has been explored as a promising site for the development of new devices used in the treatment of glaucoma. The IOP-lowering effect of more commonly used surgical approaches that use the trabecular pathway to enhance aqueous outflow is limited by episcleral venous pressure, which forms a lower limit, or “floor,” to IOP.19 Unlike in the trabecular pathway, aqueous humor outflow through the uveoscleral pathway is not limited by episcleral venous pressure, and thus it holds a greater potential for lowering IOP. Furthermore, the devices that use this pathway are free of bleb-related complications due to their blebless design. These features make this pathway an advantageous route to be used in glaucoma surgeries. GP
References
1. Wu X, Yang X, Liang Q, et al. Drugs for the treatment of glaucoma: targets, structure-activity relationships and clinical research. Eur J Med Chem. 2021;226:113842. doi: 10.1016/j.ejmech.2021.113842
2. Dick, HB, Mackert MJ, Ahmed II, et al. Two-year performance and safety results of the MINIject supraciliary implant in patients with primary open-angle glaucoma: meta-analysis of the STAR-I, II, III trials. Am J Ophthalmol. 2024;260:172-181. doi: 10.1016/j.ajo.2023.12.006
3. Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.e1. doi: 10.1016/j.ajo.2011.10.024
4. Figus M, Loiudice P, Passani A, et al. Long-term outcome of supraciliary gold micro shunt in refractory glaucoma. Acta Ophthalmol. 2022;100(3):e753-e759. doi:10.1111/aos.14989
5. Park JH, Chung HW, Yoon EG, Ji MJ, Yoo C, Kim YY. Morphological changes in the trabecular meshwork and Schlemm’s canal after treatment with topical intraocular pressure-lowering agents. Sci Rep. 2021;11(1):18169. doi: 10.1038/s41598-021-97746-x
6. American Academy of Ophthalmology. Suprachoroidal devices. EyeWiki. Accessed April 26, 2025. https://eyewiki.org/Suprachoroidal_Devices
7. Coroneo MT. Suprachoroidal drainage—centenarian progress: an inventor’s perspective. In: Francis BA, Sarkisian S Tan J, Editors, eds. Minimally Invasive Glaucoma Surgery: The Science and the Practice. Thieme; 2016:36-37.
8. Chan L, Moster M, Bicket A, et al. New devices in glaucoma. Ophthalmol Ther. 2023;12:2381–2395. doi:10.1007/s40123-023-00780-3
9. Vold S, Ahmed II, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;23(10):2103-2112
10. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without cypass micro-stent. Am J Ophthalmol. 2019;208:211-218. doi:10.1016/j.ajo.2019.07.016
11. Chan PPM, Larson MD, Dickerson JE Jr, et al. Minimally invasive glaucoma surgery: latest developments and future challenges. Asia Pac J Ophthalmol (Phila). 2023;12(6):537-564. doi:10.1097/APO.0000000000000646
12. Dervenis P, Dervenis N, Lascaratos G, Dimitriou C. Two-year data on the efficacy and safety of the miniject supraciliary implant in patients with medically uncontrolled primary open-angle glaucoma. J Clin Med. 2025;14(5):1639. doi:10.3390/jcm14051639
13. Fea A. Miniject: harnessing the drainage power of the supraciliary space. Glaucoma Today. September/October 2021. Accessed April 26, 2025. https://glaucomatoday.com/articles/2021-sept-oct/miniject-harnessing-the-drainage-power-of-the-supraciliary-space
14. Evaluation of Microinvasive Glaucoma Surgery: MINIject Versus Hydrus Microstent in Combination With Cataract Surgery (MINHY). ClinicalTrials.gov identifier: NCT06844292. Updated March 14, 2025. Accessed May 7, 2025. https://clinicaltrials.gov/study/NCT06844292
15. Denis P, Hirneiß C, Durr GM, et al. Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial. Br J Ophthalmol. 2022;106(1):65-70. doi:10.1136/bjophthalmol-2020-316888
16. Ianchulev T, Weinreb RN, Calvo EA, et al. Bio-interventional cyclodialysis and allograft scleral reinforcement for uveoscleral outflow enhancement in open-angle glaucoma patients: one-year clinical outcomes. Clin Ophthalmol. 2024;18:3605-3614. doi:10.2147/OPTH.S496631
17. Calvo E, De Francesco T, Vera L, Tyson F, Weinreb RN. Bio-interventional uveoscleral outflow enhancement surgery for primary open-angle glaucoma: 2-year results of cyclodialysis with scleral allograft reinforcement. Ophthalmol Sci. 2025;5 (4) 100727. doi: 10.1016/j.xops.2025.100727
18. Iantrek Hits 2,000 procedures for new bio-interventional technology — AlloFlo Uveo. News release. May 6, 2025. Accessed May 8, 2025. https://www.globenewswire.com/news-release/2025/05/06/3075201/0/en/Iantrek-Hits-2-000-Procedures-for-New-Bio-Interventional-Technology-AlloFlo-Uveo.html
19. Sit AJ, Aihara M, Khawaja AP, et al. Clinical implications of lowering episcleral venous pressure in the management of glaucoma and the use of Rho kinase inhibitors. Surv Ophthalmol. Published online March 17, 2025. doi:10.1016/j.survophthal.2025.03.003










