Advances in home monitoring technology are reshaping glaucoma management, offering new ways to detect intraocular pressure (IOP) fluctuations and disease progression that might otherwise go unnoticed between office visits, according to Kateki Vinod, MD. Speaking at the 2025 American Academy of Ophthalmology (AAO) glaucoma subspecialty day, Dr. Vinod described how emerging tools for home tonometry and perimetry are giving clinicians and patients a more complete view of disease dynamics.
“Home monitoring addresses a significant unmet need in glaucoma,” said Dr. Vinod, a glaucoma specialist at New York Eye and Ear Infirmary of Mount Sinai. She noted that other medical fields, such as cardiology and endocrinology, have long incorporated home blood pressure and glucose monitoring as part of standard care. “If we had more access to home monitoring during the COVID-19 pandemic, we might have been able to circumvent some of the progression that occurred when patients couldn’t come in,” she observed.
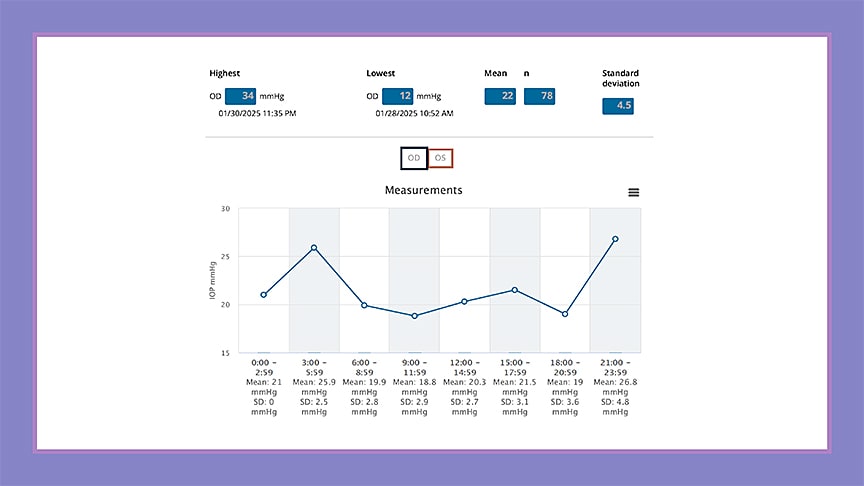
Dr. Vinod highlighted the iCare Home rebound tonometer (iCare Finland Oy), now in its second generation, which is the only device that has been approved specifically for home IOP monitoring by the US Food and Drug Administration (FDA). The device allows patients to perform IOP checks themselves without anesthesia, enabling multiple daily readings outside of traditional clinic hours. “It’s particularly helpful for identifying pressure peaks that occur late at night or early in the morning—times when we know IOP can be highest,” she said. The device has shown good correlation with Goldmann applanation tonometry,1,2 though accuracy may vary at extreme pressure levels or in eyes with very thick or thin corneas, she said.3
Despite the potential of the iCare Home2, usability remains a barrier. Although it’s generally accepted by patients, in some studies up to 30% of patients were unable to use it properly, Dr. Vinod noted.4 Still, she said, its clinical utility is clear: data from home tonometry often influences whether to adjust medications, perform laser therapy, or consider surgery, and it can help assess the success of interventions afterward (Figure 1).
Other FDA-approved and investigational technologies also show promise, said Dr. Vinod. The Sensimed Triggerfish contact lens continuously records corneoscleral circumferential changes over 24 hours, offering insight into pressure-related fluctuations during sleep, although it does not measure IOP directly. The Eyemate implantable sensor (Implandata Ophthalmic Products) provides telemetric IOP data from the suprachoroidal space or ciliary sulcus. It has been CE marked in Europe and has FDA breakthrough device designation, though it has not yet received FDA approval. “Studies published earlier this year showed that the suprachoroidal version shows good correlation to Goldmann applanation tonometry and the ciliary sulcus version may help predict RNFL progression,” noted Dr. Vinod.5,6 “So that’s exciting.”
Dr. Vinod also discussed the growing field of home perimetry, noting the rise of virtual reality (VR)–based devices that can detect functional changes outside of the office. “Although VR perimetry is a great supplement to standard automated perimetry,” she said, “the lack of standardization and validation means these tools are not quite ready to replace the gold standard.”
Combining home tonometry and perimetry may eventually allow earlier detection of disease progression, Dr. Vinod said, adding that early studies suggest that patients may find VR perimetry more user-friendly than home tonometry. She concluded by emphasizing that cost and data management remain key challenges. “Artificial intelligence is likely going to play a huge role in helping us digest home-monitoring data and synthesize it with other risk factors to deliver individualized care,” she said. GP
References
1. Realini T, McMillan B, Gross RL, et al. Test-retest reliability of intraocular pressure measurements with office-based versus home-based rebound tonometers. J Glaucoma. 2024;33(2):123-129. doi:10.1097/ijg.0000000000002441
2. Amatu JB, Bastelica P, Buffault J, Baudouin C, Labbé A. Reproducibility and reliability of intraocular pressure self-measurement with iCare HOME2 compared to Goldmann applanation tonometry. J Fr Ophthalmol. 2025;48(6):104517. doi:10.1016/j.jfo.2025.104517
3. Liu J, De Francesco T, Schlenker MB, et al. iCare Home tonometer: a review of characteristics and clinical utility. Clin Ophthalmol. 2020;14:123-130. doi:10.2147/OPTH.S284844
4. Kratz A, Zbidat R, Kishner R, Cohen M, Shalata W, Goldberg I. Assessment of the iCare HOME2, a new intraocular pressure self-measurement tonometer. J Glaucoma. 2023 Nov 1;32(11):926-929.
5. Micheletti E, Mansouri K, Dick HB, et al. Long-term safety and performance of a suprachoroidal pressure sensor system: results of the EYEMATE-SC trial follow-up study. Ophthalmology. 2025;132(7):775-784. doi:10.1016/j.ophtha.2025.01.021
6. Micheletti E, Rao H, Weinreb RN, Mansouri K; EYEMATE-IO study group. Intraocular pressure monitoring using an implantable sensor detects structural glaucoma progression in the EYEMATE-IO trial. Am J Ophthalmol. 2025;277:112-119. doi:10.1016/j.ajo.2025.05.010








