One in 8 American adults reports using a glucagon-like peptide 1 receptor agonist (GLP-1RA) medication, and prescriptions continue to increase due to these drugs’ effectiveness in reducing cardiovascular mortality and treating diabetes and obesity.1 GLP-1RAs improve blood glucose control and aid in weight loss by promoting insulin release, inhibiting glucagon secretion, slowing gastric emptying, and promoting neurohormonal satiety.2 However, GLP-1 receptors are expressed throughout the human body and are not limited to pancreatic and gastrointestinal cell types. For example, GLP-1R expression in the cardiovascular and central nervous systems is thought to, at least in part, mitigate risk of myocardial infarction, stroke, and neurodegenerative impairments.2 Due to these benefits, this class of medications is currently being investigated in clinical trials for Alzheimer disease and Parkinson disease.
The anti-inflammatory and antioxidant effects of GLP-1RAs observed in other organ systems may prevent damage in ocular tissues, leading to reduced risk of nonexudative age-related macular degeneration (AMD), exudative AMD, and primary open-angle glaucoma (POAG) compared to other diabetic medications (Figure 1).3 Extensively publicized reports of ocular adverse events, such as nonarteritic anterior ischemic optic neuropathy (NAION), have been challenging to assess due to confounding health factors and conflicting evidence. Given the vision-threatening concerns and widespread use of GLP-1RAs, some suggest candid patient conversations and closer follow-up may be warranted. Collectively, numerous retrospective and large database studies have identified potential benefits of GLP-1RA in ocular disease, indicating a need for prospective trials to more definitively identify protective effects and integrate this medication class into the ophthalmology toolbox.
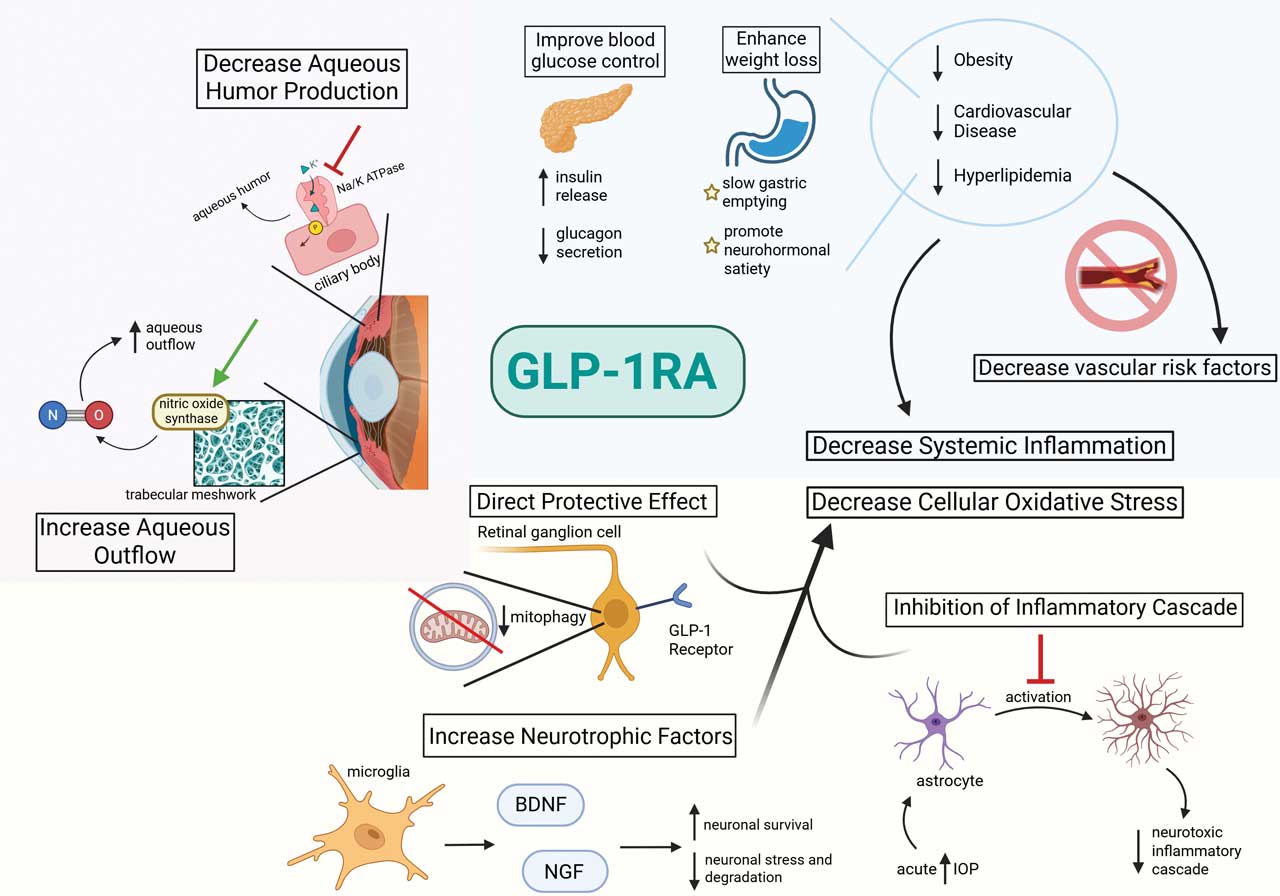
Figure 1. Proposed ocular and systemic mechanisms of glucagon-like peptide 1 receptor agonists (GLP-1RAs).
Glaucoma
Multiple retrospective large database studies have demonstrated that GLP-1RAs reduce the risk of POAG compared with insulin and other diabetes medications.3-5 Proposed mechanisms include both intraocular pressure (IOP)–lowering effects and IOP-independent effects.6
GLP-1 receptor-positive cells have been identified in human retinal ganglion cells (RGCs), which could suggest a direct protective effect of these medications at the site of damage. An additional study demonstrated that acute IOP increase causes supportive glial cells to become proinflammatory and neurotoxic. This effect is blocked by GLP-1RA, which prevents optic nerve axonopathy.7-9 GLP-1 signaling has been shown to promote microglia-mediated expression of neurotrophic factors, such as brain-derived neurotrophic factor and nerve growth factor, to promote neuronal survival and inhibit stress pathways in neurodegenerative conditions.10,11
Regarding IOP-lowering mechanisms, GLP-1RAs have been shown to inhibit sodium-potassium ATPase in the choroid plexus, decreasing cerebrospinal fluid secretion and lowering intracranial pressure. It is postulated these medications may similarly reduce IOP by inhibiting sodium-potassium ATPase–mediated aqueous humor secretion by the ciliary body.9,12 Additionally, GLP-1RAs activate nitric oxide (NO) synthase expressed in Schlemm’s canal and the trabecular meshwork, increasing NO production to reduce trabecular meshwork contraction, improving aqueous outflow.13 More research is needed to delineate the effects of GLP-1RAs in the various glaucoma subtypes, including neovascular glaucoma.
Diabetic Retinopathy and Diabetic Eye Disease
Typically thought of as a retinal microvascular disease, diabetic retinopathy (DR) is also a retinal neurodegenerative process.14-17 GLP-1RAs decrease RGC damage by preventing mitophagy in diabetic rat models and, in vitro, demonstrating the neuroprotective effects of these medications on the retina.18
There have been mixed results from published clinical studies. One large electronic database study showed there was a significantly increased risk of diabetic macular edema following GLP-1RA initiation compared to sodium-glucose cotransporter 2 inhibitors (SGLT2i).19 A separate meta-analysis of 4 major clinical trials suggested prolonged use of GLP-1RA was associated with an elevated risk of rapidly worsening DR.20 However, a retrospective single-institution study showed that although other anti-diabetic medications (such as sodium-glucose cotransporter 2 inhibitors) were associated with a lower risk of sight-threatening DR, GLP-1RAs were not shown to confer any increased DR risk, clinical worsening, or increased intervention in DR.21,22
Although clear protective benefits in diabetic retinopathy development and impact on disease progression remain under investigation, GLP-1RAs have been shown to reduce complications from chronic diabetes, such as retinal vein occlusion and cardiovascular disease.23,24
Age-Related Macular Degeneration
Nonexudative age-related macular degeneration (AMD) is a complex and multifaceted disease caused by inflammation, lipid deposition, and oxidative stress, leading to drusen deposition and retinal pigment epithelium (RPE) damage.25 This damage culminates in geographic atrophy or disruption of Bruch’s membrane, leading to severe vision loss and, in some cases, neovascularization. In agreement with the profound anti-inflammatory effects of GLP-1RAs, a recent study demonstrated a protective effect of these medications against the development of non-exudative AMD compared with other diabetes and lipid-lowering medications.3
GLP-1RAs have also been shown to alter cellular metabolism to mitigate oxidative stress, which is thought to play a pivotal role in AMD pathogenesis.26 It is also feasible that reduction in AMD risk factors such as obesity, cardiovascular disease, and hyperlipidemia with GLP-1RAs could further provide benefit by reducing systemic inflammation and vascular risk factors.27-30 GLP-1RAs were also associated with reduced hazard in exudative AMD compared with insulin, aspirin, and statins.3
Future studies are needed to determine whether this class of medications inhibits progression from nonexudative to neovascular or late atrophic stages of AMD.
Adverse Effects
Aside from the debated increased risk of DR progression, a separate complication that has garnered widespread attention is the purported association of semaglutide with an increased risk of NAION, a vision-threatening condition.31 However, a causal relationship has not been established.32 Furthermore, subsequent large retrospective studies have demonstrated no effect of GLP-1RA on the increased risk of ischemic optic neuropathy.33,34 However, these studies are limited by the inability to identify NAION with electronic health record coding with absolute certainty.
One potential mechanism could be increased prevalence of NAION risk factors in patients starting GLP-1RA or that sudden and rapid decline in blood glucose could increase the risk of diabetic papillopathy or worsened DR.31,35-37 Prospective trials are needed to further explore this potential adverse event and worsened DR, which is the central premise of the ophthalmic FOCUS trial that is expected to release results in 2026. Otherwise, current recommendations are for patients to be screened for baseline diabetic retinopathy before initiating GLP-1RA and participate in personalized risk discussions.
Clinical Recommendations and Future Research
Numerous retrospective studies have outlined the potential benefits of GLP-1RA on ocular disease; however, this must be balanced with additional studies suggesting ophthalmic complications from this medication class. Prospective trials are needed to further define potential protective effects of these medications in glaucoma and AMD. Additionally, with equivocal findings in DR and NAION, discussing personalized ocular risk of starting GLP-1RA in patients is key, and considering increased follow-up frequency after medication initiation may be warranted. GP
References
1. Harris E. Poll: roughly 12% of US adults have used a GLP-1 drug, even if unaffordable. JAMA. 2024;332(1):8. doi:10.1001/jama.2024.10333
2. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(6):740-756. doi:10.1016/j.cmet.2018.03.001
3. Allan KC, et al. Glucagon-like peptide-1 receptor agonist impact on chronic ocular disease including age-related macular degeneration. Ophthalmology. 2025;S0161-6420(25)00070-3. doi:10.1016/j.ophtha.2025.01.016
4. Niazi S, et al. Association between glucagon-like peptide-1 receptor agonists and the risk of glaucoma in individuals with type 2 diabetes. Ophthalmology. 2024;131(9):1056-1063. doi:10.1016/j.ophtha.2024.03.004
5. Sterling J, et al. Glucagon-like peptide 1 receptor agonist use is associated with reduced risk for glaucoma. Br J Ophthalmol. 2023;107:215-220. doi:10.1136/bjophthalmol-2021-319232
6. Sterling JK, et al. GLP-1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. 2020;33(4):108271. doi:10.1016/j.celrep.2020.108271
7. Hebsgaard JB, et al. Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes Metab. 2018;20(9):2304-2308. doi:10.1111/dom.13339
8. Lawrence ECN, et al. Topical and systemic GLP-1R agonist administration both rescue retinal ganglion cells in hypertensive glaucoma. Front Cell Neurosci. 2023;17:1156829. doi:10.3389/fncel.2023.1156829
9. Quaranta L, et al. Glaucoma and neuroinflammation: an overview. Surv Ophthalmol. 2021;66:693-713. doi:10.1016/j.survophthal.2021.02.003
10. Salcedo I, et al. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166(5):1586-1599. doi:10.1111/j.1476-5381.2012.01971.x
11. Andreasen CR, et al. How glucagon-like peptide 1 receptor agonists work. Endocrinol Connect. 2021;10(7):200-212. doi:10.1530/EC-21-0130
12. Botfield HF, et al. A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med. 2017;9(410):eaai8462. doi:10.1126/scitranslmed.aan0972
13. Han B, et al. The application of nitric oxide for ocular hypertension treatment. Molecules. 2021;26(17):7306. doi:10.3390/molecules26237306
14. Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283-290. doi:10.1016/S0278-5846(03)00023-X
15. Sohn EH, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113(19):E2655–E2664. doi:10.1073/pnas.1522014113
16. Rajagopal R, et al. Functional deficits precede structural lesions in mice with high-fat diet-induced diabetic retinopathy. Diabetes. 2016;65(4):1072-84. doi:10.2337/db15-1255
17. Batista AF, et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol. 2018;245(1):85-100. doi:10.1002/path.5056
18. Zhou HR, et al. Neuroprotective role of GLP-1 analog for retinal ganglion cells via PINK1/Parkin-mediated mitophagy in diabetic retinopathy. Front Pharmacol. 2021;11:589114. doi:10.3389/fphar.2020.589114
19. Wai KM, et al. Impact of GLP-1 agonists and SGLT-2 inhibitors on diabetic retinopathy progression: An aggregated electronic health record data study. Am J Ophthalmol. 2024;265:39-47. doi:10.1016/j.ajo.2024.04.010
20. Yoshida Y, et al. Progression of retinopathy with glucagon-like peptide-1 receptor agonists with cardiovascular benefits in type 2 diabetes: a systematic review and meta-analysis. J Diabetes Complications. 2022;36(8):108255. doi:10.1016/j.jdiacomp.2022.108255
21. Barkmeier AJ, et al. Comparative effectiveness of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase-4 inhibitors, and sulfonylureas for sight-threatening diabetic retinopathy. Ophthalmol Retina. 2024;8(10):943-952. doi:10.1016/j.oret.2024.05.003
22. Joo JH, et al. The effect of glucagon-like peptide-1 receptor agonists on diabetic retinopathy at a tertiary care center. Ophthalmol Sci. 2022;4(6):100547. doi:10.1016/j.xops.2024.100547
23. Pan SY, et al. Risk of retinal vein occlusion between GLP-1 receptor agonists and DPP-4 inhibitors in type 2 diabetes: a retrospective cohort study. Ophthalmol Sci. 2022;5(4):100734. doi:10.1016/j.xops.2025.100734
24. Muayad J, et al. Influence of common medications on diabetic macular edema in type 2 diabetes mellitus. Ophthalmol Retina. 2025;9(6):505-514. doi:10.1016/j.oret.2024.12.006
25. Hernández C, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2016;65(1):172-187. doi:10.2337/db15-0443
26. Ruan Y, Jiang S, Gericke A. Age-related macular degeneration: role of oxidative stress and blood vessels. Int J Mol Sci. 2021;22(3):1296. doi:10.3390/ijms22031296
27. Fleckenstein M, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. doi:10.1038/s41572-021-00265-2
28. Ledesma-Gil G, et al. Subretinal drusenoid deposits are strongly associated with coexistent high-risk vascular diseases. BMJ Open Ophthalmol. 2022;7(1):e001154. doi:10.1136/bmjophth-2022-001154
29. Zhang QY, et al. Overweight, obesity, and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(3):1276-1283. doi:10.1167/iovs.15-18637
30. Li B, Goss D, Miller JW. Systemic dyslipidemia in age-related macular degeneration: an updated systematic review and meta-analysis. Ophthalmology Sci. 2024;4:100147. doi:10.1016/j.xops.2023.100341
31. Hathaway JT, et al. Risk of nonarteritic anterior ischemic optic neuropathy in patients prescribed semaglutide. JAMA Ophthalmol. 2024;142(8):732-739. doi:10.1001/jamaophthalmol.2024.2296
32. Lin JB, Tsubota K, Apte RS. A glimpse at the aging eye. NPJ Aging Mech Dis. 2016;2:16003. doi:10.1038/npjamd.2016.3
33. Abbass NJ, et al. The effect of semaglutide and GLP-1 RAs on risk of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2025;274:24-31. doi:10.1016/j.ajo.2025.02.025
34. Chou CC, et al. Association between semaglutide and nonarteritic anterior ischemic optic neuropathy: a multinational population-based study. Ophthalmology. 2025;132(4):381-388. doi:10.1016/j.ophtha.2024.10.030
35. Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye. 2015;29(1):65-79. doi:10.1038/eye.2014.144
36. Hebsgaard JB, et al. Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes Metab. 2018;20(9):2304-2308. doi:10.1111/dom.13339
37. Vilsbøll T, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889-897. doi:10.1111/dom.13172












