A measure of how retinal blood flow adapts to changing conditions may offer a new way to detect early glaucoma, according to research presented at the annual meeting of the American Glaucoma Society (AGS). Dynamic assessment of vascular reactivity in the optic nerve head revealed differences between control patients and those with early glaucoma, suggesting that impaired autoregulation precedes structural damage and may serve as an early biomarker for disease.
Saige Oechsli, a clinical research assistant in the department of ophthalmology and visual sciences at the University of Maryland School of Medicine, discussed the findings on behalf of a research team led by Osamah Saeedi, MD, whose work focuses on ocular blood flow and glaucoma pathophysiology.
“The problem that we all see in our clinics is that, despite treatment—even when intraocular pressure is well controlled—patients still show a considerable amount of progression,” Oechsli said, noting that traditional IOP-based monitoring remains insufficient to capture all risk. She pointed to prior epidemiologic studies, such as the Barbados Eye Study1 and the Baltimore Eye Survey,2 which linked systemic hemodynamic factors to glaucoma risk, and framed vascular autoregulation as an underexplored pathophysiological feature (Figure 1).
Figure 1. Vascular mechanisms in glaucoma, proposed in the late 19th century by British ophthalmologist Priestley Smith and supported by epidemiologic associations with systemic blood pressure, include reduced baseline ocular blood flow and impaired autoregulation, as demonstrated in the current study.
The University of Maryland team conducted a cross-sectional interventional study using laser speckle contrast imaging to quantify blood flow at the optic nerve head in 3 groups: control participants, preperimetric glaucoma suspects, and patients with mild primary open-angle glaucoma (POAG). “Our purpose was to assess differences in autoregulation of blood flow control, comparing pre-perimetric glaucoma, mild glaucoma, and healthy controls,” Oechsli said.
Participants breathed 100 percent oxygen through a nonrebreather mask for 10 minutes while retinal blood flow was measured at baseline and under induced hyperoxia conditions. Decreases in flow with hyperoxia indicate normal vascular reactivity, as vessels constrict in response to increased oxygen. In control patients, mean blood flow decreased by more than 10% under hyperoxia. In contrast, eyes with mild POAG demonstrated a smaller decrease, suggesting reduced autoregulatory responsiveness. “Hyperoxia is used as an indicator of vascular reactivity, which serves as a measure of autoregulatory blood flow,” she explained.
In addition to mean flow reductions, the study assessed secondary indices such as resistivity index and volumetric skew, dynamic parameters that respond to changes in metabolic demand and vascular resistance. Control participants exhibited larger changes in these metrics compared with glaucoma patients, indicating more robust autoregulatory capacity. Among glaucoma suspects, some measures more closely resembled early glaucoma than controls, raising the possibility that altered autoregulation may appear before measurable visual field damage.
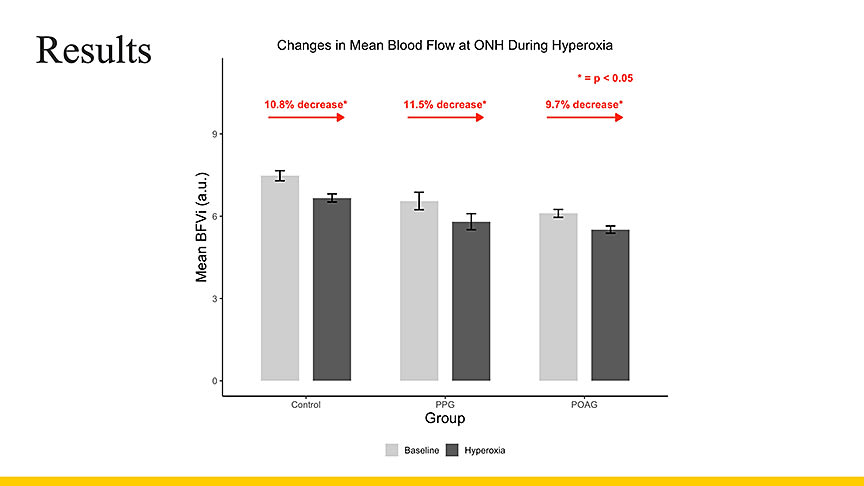
Figure 2. Baseline optic nerve head blood flow was highest in controls and lowest in eyes with primary open-angle glaucoma, and during induced hyperoxia, controls and preperimetric glaucoma eyes showed larger reductions in mean flow than mild primary open-angle glaucoma eyes, consistent with impaired autoregulation in glaucoma.
Demographically, the analysis included 226 eyes from 126 patients with a mean age in the mid-60s. Oechsli emphasized that all diagnoses were verified by a glaucoma specialist. In baseline comparisons, the research team observed a “consistent decrease in baseline flow when comparing groups,” with lowest baseline values in the mild glaucoma cohort. She noted that the pattern of response under hyperoxia further distinguished glaucomatous from nonglaucomatous eyes: “Controls and glaucoma suspects showed a greater response. The glaucoma group showed a smaller change” (Figure 2).
The findings are aligned with earlier work3-5 suggesting that retinal blood flow regulation is altered in glaucoma (Figure 3). By quantifying autoregulatory responses, researchers hope to identify a physiological marker that can supplement structural measures such as OCT or functional measures such as perimetry. “Patients with early and pre-perimetric glaucoma showed lower baseline optic nerve head blood flow compared with controls,” Oechsli said, “suggesting potential utility as an early-stage marker.”
Figure 3. Altered retinal blood-flow autoregulation is detectable early in glaucoma, supporting dynamic flow measures as potential biomarkers for early disease detection.
The AGS meeting presentation was originally scheduled to be given by Yash Porwal, another clinical research assistant at the University of Maryland School of Medicine, but travel problems prevented him from arriving in time. Following the presentation, Dr. Saeedi acknowledged his role in the study. GP
References
1. Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112(6):821-829. doi:10.1001/archopht.1994.01090180121046
2. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open-angle glaucoma among White and Black Americans: the Baltimore Eye Survey. Arch Ophthalmol.1991;109(8):1090-1095. doi:10.1001/archopht.1991.01080080050026
3. Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. a population-based assessment. Arch Ophthalmol. 1995;113(2):216-221. doi:10.1001/archopht.1995.01100020100038
4. Vinnett A, Kandukuri J, Le C, Cho KA, et al. Dynamic alterations in blood flow in glaucoma measured with laser speckle contrast imaging. Ophthalmol Glaucoma. 2022;5(3):250-261. doi:10.1016/j.ogla.2021.10.005
5. Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99(1):137-143. doi:10.1001/archopht.1981.03930010139020








